Subxiphoid single-port video-assisted thoracoscopic surgery
Introduction
Thoracoscopic surgery is increasingly performed because of improvements in endoscopic instruments and surgical techniques. Growing evidence indicates that thoracoscopic surgery is associated with decreased postoperative pain, reduced length of hospital stay, and fewer postoperative complications (1-6). However, traditional transthoracic video-assisted thoracic surgery (VATS) always involve the creation of a small incision on the chest wall and may result in intercostal neuralgia and chronic thoracotomy pain (7-9). We believe that the approach or incision in thoracic surgery still has the potential for continuous improvements. Therefore, we investigated an alternative approach for thoracic intervention.
Subxiphoid incision is employed in many transthoracic procedures for both thoracic and cardiovascular surgeries. These procedures include subxiphoid pericardial window creation, coronary surgery, thymectomy, and pulmonary metastasectomy (10-14). We have used animal models to determine alternative approaches such as natural orifice transluminal surgery through transoral, transtracheal (15-18), and transumbilical routes (19). However, most of these procedures are currently not feasible to be performed in humans.
With previous experience on transthoracic single-port VATS surgery since 2010 (20,21), despite trauma outside on the chest has been minimized to only one. However, we observed that the single-port approach did not significantly reduce postoperative pain as compared with a two-port approach (22). Moreover, this surgery still leaves a wound in the chest near the breast, which is not idealistic especially for young females with cosmetic concern. Therefore, we switched to a subxiphoid single-port technique for lung resection and described the first case report of subxiphoid single-incision thoracoscopic left upper lobe (LUL) lobectomy (23). However, this novel technique is quite challenging and has some limitations. Appropriate patient selection and modification of instruments are necessary to perform a safe and sound surgery. Here, we report our preliminary experience with the subxiphoid single-incision approach for thoracoscopic chest surgery.
Materials and methods
This study retrospectively analyzed 39 consecutive patients who underwent subxiphoid single-incision thoracoscopic surgery at the Koo Foundation Sun Yat-Sen Cancer Center between December 2013 and January 2016. Inclusion criteria for patients were (I) confirmed early lung cancer without obvious mediastinal lymph node (LN) metastasis according to preoperative image staging, (II) metastatic lung cancer, (III) mediastinal disease, (IV) and unconfirmed lung nodule or benign lung lesion that deserved surgery. Exclusion criteria were similar to those for the traditional transthoracic approach. Moreover, patients with clinical stage I disease that is suitable for technique-demanding atypical segmentectomy, e.g., lower lobe posterior basal segmentectomy, were suggested to undergo transthoracic sublobar resection instead of subxiphoid lobectomy to preserve more lung parenchyma. All the patients were carefully explained about the benefits and drawbacks of the subxiphoid approach versus the transthoracic approach and were asked to sign an informed consent for subxiphoid surgery. Preoperative workup included complete blood counts, serum biochemistry tests, chest computed tomography (CT) scans, and positron emission tomography computed tomography (PET-CT) scans. Information on demographic characteristics of patients, operating time, number of dissected LNs, length of hospital stay, postoperative complications, and pathological characteristics of tumors according to the American Joint Committee on Cancer staging system (7th edition) was collected. This study was approved by the institutional ethical committee in Sun Yat-Sen Cancer Center Hospital, and we obtained the informed consent from every patient before surgery was performed.
Operative setting and surgical technique
The patients were orotracheally intubated with a double-lumen endotracheal tube to provide adequate one lung ventilation. Positioning of the patients was based on the location of the lesion and the procedure to be performed. Supine position was the standard position for mediastinal surgery, such as thymomectomy, or bilateral surgery for bilateral pneumothorax or pulmonary metastases. Semi-decubitus position was adopted in the early few cases; however, we now prefer true lateral-decubitus position as the standard position for anatomic lung resection. Artificial CO2 pneumothorax was applied to none of the patients in the whole series.
Creation of the subxiphoid single port
A 3- to 4-cm vertical skin incision was made above the xiphoid process, after cutting the skin and the linea alba, and the muscle was detached to reach the xiphoid process. A blunt dissection was performed using a finger to create a subxiphoid route similar to a retrosternal route used for gastric tube pull-up after esophagectomy. A deep thyroid retractor was then applied to lift the sternum. By holding a sucker in the left hand, and an endoscopic hook cautery in the right hand of the operator, a thoracoscope was inserted into the substernal tunnel created previously by finger dissection. Under thoracoscopic guidance, pericardial fat was detached from the pleura and pericardium and the thoracoscope was inserted into the pleural cavity after hook cauterization on the pleura under direct observation (Figure 1A,B). A homemade trauma-less wound protector (trocar set) was designed for the subxiphoid port. By partially inserting an extra small size Alexis Wound Retractor (Applied Medical, USA) into a 12-mm laparoscopic trocar (Endopath Xcel Bladeless Trocars; Ethicon Inc., Blue Ash, OH, USA; Figure 1C), the wound protector was deployed into the pleural cavity. This was confirmed later by using the thoracoscope. Finally, a sternal lifter was used to lift the tunnel above the mediastinum and the pericardium to create more working space as well as avoid the interference of the heartbeat. A 10-mm, zero-degree articulating thoracoscope (EndoEye Flex; Olympus, Tokyo, Japan) was inserted into the subxiphoid incision to explore the pleural cavity for anatomic lung resection (Figure 1D).
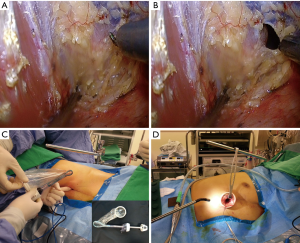
Anatomic consideration and surgical technique
When the traditional 30-degree scope was inserted through the subxiphoid port into the chest cavity, it ran parallel to the mediastinum and directly faced the oblique fissure. It is even clear if we apply 3D-reconstructed CT images for better anatomic understanding; the subxiphoid incision itself is located at the horizontal level of the fifth costal junction to the sternum, and the distance might be 2–3 cm longer compared with a transintercostal approach. With the oblique fissure in the front, it was easy to visualize the interlobar bronchovascular structure from the oblique fissure. The anterior and apical pulmonary arteries and the superior/inferior pulmonary vein (Figure 2) could also be easily approached anteriorly/inferiorly (Figure 2D); however, it was quite difficult to reach the deeply seated left-side subcarinal area. The instrument-fighting problem during the subxiphoid single-port surgery would be even more challenging than that encountered during the transthoracic approach since the mediastinum occupied the lower part of the working tunnel, which reduced the working space. Moreover, there was interference related to instrument transmission from the heartbeat during left-side procedures. Therefore, we employed the following methods to minimize these problems: (I) we released the fibrous and adipose tissue around the subxiphoid tunnel away from the pericardium and diaphragm. This enlarged the space of the port and provided more room for manipulation; (II) we applied a sternal retractor to lift the port away from the mediastinum, including the pericardium; (III) we used a small tidal volume whenever possible to let the mediastinum drop into the dependent non-operative side; (IV) we used long and curved or articulated instruments (Figure 2A-C) to avoid contact with the heart and to minimize both internal and external instrument fighting; (V) we used an articulate scope to obtain a better view from above, which was similar to the view with the traditional 30-degree lens in the transthoracic approach, and also minimized the instrument fighting.
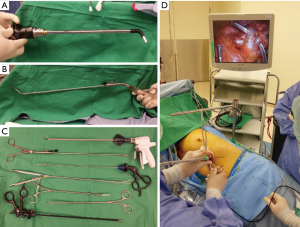
Technically, the subxiphoid approach for anatomic lung resection follows the same principle as the transthoracic approach (Figure 3A), i.e., it uses a combination of traditional laparoscopic instruments or double-joint thoracoscopic instruments (Scanlan International, Inc., USA) for exposure and dissection, Endostapler (Ethicon Inc.), or Endo GIA Curved Tip Reload (Covidien and Medtronic, USA) for transecting the bronchovascular structure (Figure 3B) and lung parenchyma (20,21). Traditional open chest instruments are no longer used. However, stapling of vessels might be more challenge than transthoracic procedures related to instrument fighting and different angles needed to apply to the envil to pass through the hilar vessel safely.

For LN dissection, the intrapulmonary, hilar, and/or mediastinal LNs were retrieved using a combination of laparoendoscopic instruments and Harmonic scalpel (Ethicon Inc.) and the representative hilar/segmental LNs were checked intraoperatively by frozen to exclude the possibility of nodal metastasis. Complete lymphadenectomy in en-bloc style for the deeply seated right-side upper mediastinum and the left-side subcarinal nodes was sometimes difficult not only because most of the energy-delivering instruments, such as hook and Harmonic scalpel, are straight and parallel to the mediastinum, but also due to the potential bleeding risks if injured the nearby great vessels. In some patients, right subcarinal lymphadenectomy is possible, but only when performed by experienced surgeons. Therefore, it is extremely important to carefully select patients suitable for this approach, which is probably reserved for nodal-negative patients after preoperative staging workup. The present study used both systematic LN sampling according to ACOSOG Z0030 protocol (24) and/or lobe-specific dissection method (25-27) based on the lobar location of the lesion. For patients with lung cancers having pure, ground-glass opacity (GGO), and only regional LN sampling was performed.
The specimen was secured in a plastic bag and delivered through the subxiphoid incision (Figure 3C). Specimen retrieval from the subxiphoid port was much easier than that through the transthoracic port without the limitation of the rib cage, thus indicating an advantage of the subxiphoid approach over the transthoracic approach. A chest tube was placed in the pleural cavity through the same subxiphoid wound (28,29). No epidural pain control or intercostal blockade was used in this study.
Results
From December 2013 to January 2016, 39 patients underwent 40 chest surgeries via a single incision 3- of 4-cm in length on the subxiphoid area (Table 1). In one patient, the surgery had to be repeated because of single pulmonary metastasis 1 year and 10 months after the previous bilateral pulmonary metastasectomies. There were 29 females and 10 males, with a median age of 55 years (range, 33–69 years). Indication for surgery included 24 patients with primary lung cancers, eight with lung metastases, two with benign lung lesions, one with bilateral pneumothorax, and five with mediastinal tumors. Operative procedures included lobectomy in 21, segmentectomy in five, wedge resection in nine, and mediastinal surgery in five patients. There were two patients requiring an additional 3-cm single chest incision to complete the procedure due to inadequate length of instruments and anthracotic LNs for anatomic lung resection. Bilateral surgery was adopted for four patients (three bilateral multiple wedge resections and another one patient underwent right-side upper mediastinal LN sampling to rule out N3 metastasis, followed by a left lower lobectomy and LN sampling). For 24 patients with primary lung cancers, the median tumor size was 2.0 cm (range, 0.8–5.5 cm), the median operation time was 3 hours (range, 1.0–4.5 hours), the median blood loss was 30 cc (range, 10–150 cc), and the median LN dissection number was 16.0 only (range, 4–37), which was inferior to our historical control group in the transthoracic procedure (20-22). Furthermore, fragmentation of dissected LNs was observed more frequently in the subxiphoid group. Tumor stages based on AJCC 7th were stage I in 18, stage IIa in 4, stage IIIa in 1, and stage M1a (two tiny pleural seedlings) in 1. For five patients with mediastinal surgery, radical thymothymecotmy was performed in one, resection of diaphragmatic bronchogenic cyst in one, and three patients received thymomectomy, with one of them undergoing partial pericardial resection and mesh-repair through the single subxiphoid incision. Among these 39 patients, 26 underwent anatomic lung resections [seven right upper lobe (RUL)/five right middle lobe (RML)/two right lower lobe (RLL)/four LUL/three left lower lobe (LLL) lobectomies, two tri-segmentectomies, two S3-segmentectomies, and one common basal segmentectomy], with a median operation time of 3 h (range, 2.0–4.5 h), median blood loss of 30 cc (range, 10–170 cc), and a median hospital stay of 5 days (range, 4–10 days).
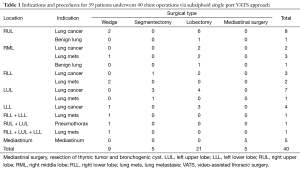
Full table
No surgical mortality was observed in the entire series. Complications (4/40, 10.0%) included transient arrhythmia in the early learning curve period in two patients. Postoperative chylothorax was observed in one patient, which was managed with thoracic duct ligation. The procedure for this particular patient was right-side lymphadenectomy (station 4R) and wedge resection under the indication for second primary GGO lung cancer 2.5 years after the previous surgery for left upper lung cancer. One patient developed postoperative bleeding on postoperative day 2 because of delayed bleeding from a small vessel in the pericardial fat. This patient was reoperated using the transthoracic single-port approach to check the bleeding. After this painful experience, we innovated the current method for the safe creation of a subxiphoid port under direct vision. None of the patients experienced herniation in the subxiphoid area, nor phrenic nerve palsy, in this study.
Discussion
Based on our previous experience with the transthoracic single-port approach (20-22) and review of current animal and human studies (15-19), we employed the subxiphoid single-port approach for anatomic lung resection and learned a lot from this novel procedure. Although both the transthoracic and subxiphoid single-port techniques focus on hilar dissection, port design, instrument setting, and limitations of both the techniques vary considerably. We encountered some difficulties while using the subxiphoid single-port approach. First, creation of a safe subxiphoid single port is crucial. In contrast to the use of a laparoscopic trocar or SILS™ Port (Covidien) in other studies (13,14), we used a wound protector for creating the subxiphoid incision, which was easier and similar to that created in the transthoracic approach. Second, the visual field is restricted by the standard 30-degree thoracoscope inserted through the subxiphoid single incision because the visual field is parallel to the mediastinum and is disrupted by the instruments. An articulating endoscope (Figure 2A) is more useful in regaining the visual field similar to that in the traditional transthoracic approach and provides more space for other surgical instruments. Third, instrumental transmission of the heartbeat is troublesome, especially during dissection or stapling of the hilar structures while performing procedures on the left side. Moreover, instruments compressing the heart might induce arrhythmia and even transient hypotension. We used a sternal lifter and applied low tidal volume setting on the ventilator to overcome this problem. Fourth, traditional endoscopic instruments might be too short and interference between the instruments might be problematic during surgery, especially in tall patients, as experienced in this study. Therefore, long and articulating endoscopic instruments might be helpful. Fifth, structures in the posterior aspect of the chest, e.g., the subcarinal area, are difficult to approach with a patient in the supine position without the availability of proper instruments for retraction. Thus, proper positioning of the patient is important. In our experience, the supine position was suitable for bilateral pulmonary metastasectomy of tumors located in the anterior chest. The true decubitus position was suitable for anatomic lung resection with or without mediastinal LN dissection. Finally, mastering this technique requires a learning curve. This technique is easier to perform for surgeons having a previous experience with single-port transthoracic techniques.
We considered other approaches such as subcostal approach; however, this approach still requires cutting the external oblique muscle. Moreover, intercostal nerve branches attached to the subcostal area still remain, which may induce neuralgia and pain. Furthermore, the distance to the target hilar structure is farther than subxiphoid. With respect to patient recovery after surgery, subjective wound pain after surgery was considerably reduced after the subxiphoid approach compared with that after the transthoracic approach. Mediastinum pain was minor and was easily controlled with a short-term use of painkillers. No limitations were observed in shoulder movements immediately after the surgery, and 90% (36/40) of the patients did not require any painkillers at their first return to the clinic compared with the vast majority who still asked for painkiller refill in the transthoracic group.
This study suggests that anatomic resection of every lobe and traditional segmentectomy, including trisegmentectomy and basilar segmentectomy, could be performed using the subxiphoid single-incision approach, and modification of the technique and instrument settings is crucial to the success of this approach. Radicalism for mediastinal LN dissection for lung cancer was inferior to transthoracic procedures in our historical control group (20-22).
The main limitation of our study is the limited number of patients. We explained in detail the benefits and potential risks of the subxiphoid single-incision approach to each patient included in the study and offered single-incision transthoracic thoracoscopic surgery at the same time. About half of the patients hesitated and declined to undergo the subxiphoid approach because they felt that it is still new and may be associated with inferior lymphadenectomy results. Another consideration was for segmentectomy, which is much easier through the transthoracic approach than through the subxiphoid approach. We think that subxiphoid segmentectomy is technically more challenging than transthoracic procedures, particularly when handling atypical segmentectomy. During the study period, most patients were recommended to undergo the transthoracic sublobar resection if lobectomy via the subxiphoid approach could be avoided. At present, we intend to enroll patients with cT2N0M0 or centrally located cT1N0M0 cancers for subxiphoid lobectomy; subxiphoid segmentectomy was reserved for traditional segmentectomy, as lingular or trisegmentectomy, superior segmentectomy of the lower lobe.
Conclusions
In conclusion, we observed that anatomic lung resection through lobectomy or segmentectomy is feasible through the subxiphoid single-incision approach. This approach is associated with advantages of decreased postoperative pain, better cosmetic outcome, and easy specimen retrieval as compared with the traditional transthoracic approach. However, this novel approach has limitations for mediastinal LN dissection and handling unexpected intraoperative complications such as major bleeding or anthracotic LNs. The future looks promising with the possibility of designing novel single-port or robotic single-port instruments for this approach. However, large-scale studies are needed to document the application and to compare clinical outcomes associated with the subxiphoid single-incision approach with those associated with the transthoracic approach to provide evidence of the benefits of the former approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethical committee in Sun Yat-Sen Cancer Center Hospital and written informed consent was obtained from all patients.
References
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Sihoe AD, Cheung CS, Lai HK, et al. Incidence of chest wall paresthesia after needlescopic video-assisted thoracic surgery for palmar hyperhidrosis. Eur J Cardiothorac Surg 2005;27:313-9. [Crossref] [PubMed]
- Yim AP, Liu HP. Complications and failures of video-assisted thoracic surgery: experience from two centers in Asia. Ann Thorac Surg 1996;61:538-41. [Crossref] [PubMed]
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Liberman M, Labos C, Sampalis JS, et al. Ten-year surgical experience with nontraumatic pericardial effusions: a comparison between the subxyphoid and transthoracic approaches to pericardial window. Arch Surg 2005;140:191-5. [Crossref] [PubMed]
- Watanabe G, Yamaguchi S, Tomiya S, et al. Awake subxyphoid minimally invasive direct coronary artery bypass grafting yielded minimum invasive cardiac surgery for high risk patients. Interact Cardiovasc Thorac Surg 2008;7:910-2. [Crossref] [PubMed]
- Hsu CP, Chuang CY, Hsu NY, et al. Subxiphoid approach for video-assisted thoracoscopic extended thymectomy in treating myasthenia gravis. Interact Cardiovasc Thorac Surg 2002;1:4-8. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Suda T, Ashikari S, Tochii S, et al. Single-incision subxiphoid approach for bilateral metastasectomy. Ann Thorac Surg 2014;97:718-9. [Crossref] [PubMed]
- Liu YH, Chu Y, Wu YC, et al. Natural orifice surgery in thoracic surgery. J Thorac Dis 2014;6:61-3. [PubMed]
- Liu CY, Chu Y, Wu YC, et al. Transoral endoscopic surgery versus conventional thoracoscopic surgery for thoracic intervention: safety and efficacy in a canine survival model. Surg Endosc 2013;27:2428-35. [Crossref] [PubMed]
- Liu YH, Chu Y, Liu CY, et al. Feasibility of the transtracheal approach for the thoracic cavity in a large animal model. Surg Endosc 2011;25:1652-8. [Crossref] [PubMed]
- Liu YH. Natural orifice transluminal endoscopic surgery: a transtracheal approach for the thoracic cavity in a live canine model. J Thorac Cardiovasc Surg 2011;141:1223-30. [Crossref] [PubMed]
- Wen CT, Chu Y, Yeh CJ, et al. Feasibility and safety of endoscopic transumbilical thoracic surgical lung biopsy: a survival study in a canine model. J Surg Res 2013;183:47-55. [Crossref] [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Liu CY, Cheng CT, Wang BY, et al. Number of Retrieved Lymph Nodes and Postoperative Pain in Single-incision and Multiple-incision Thoracoscopic Surgery. Ann Surg 2015. [Epub ahead of print]. [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010;139:1001-6. [Crossref] [PubMed]
- Guizilini S, Bolzan DW, Faresin SM, et al. Pleurotomy with subxyphoid pleural drain affords similar effects to pleural integrity in pulmonary function after off-pump coronary artery bypass graft. J Cardiothorac Surg 2012;7:11. [Crossref] [PubMed]
- Guden M, Korkmaz AA, Onan B, et al. Subxiphoid versus intercostal chest tubes: comparison of postoperative pain and pulmonary morbidities after coronary artery bypass grafting. Tex Heart Inst J 2012;39:507-12. [PubMed]
Cite this article as: Liu CC, Shih CS, Liu YH, Cheng CT, Melis E, Liu ZY. Subxiphoid single-port video-assisted thoracoscopic surgery. J Vis Surg 2016;2:112.


