Preoperative accuracy of gastric cancer staging in patient selection for preoperative therapy: race may affect accuracy of endoscopic ultrasonography
Introduction
Over the last 15 years, large randomized controlled studies have validated the benefit of preoperative therapy for patients with resectable gastric cancer (1-4). Preoperative staging using computed tomography (CT) and endoscopic ultrasonography (EUS) is used to select patients for preoperative treatment (5,6). Patients are generally considered good candidates for preoperative therapy if they have clinical stage T2 or higher tumors or positive lymph nodes. If preoperative staging is inaccurate, patients with early-stage gastric cancer may receive inappropriate preoperative treatment that delays surgical intervention. Conversely, patients with advanced gastric cancer may miss out on beneficial preoperative treatment if their disease is understaged. Several reports and meta-analyses regarding the accuracy of preoperative EUS T staging have been published (7-10), but the reported accuracies differed substantially (8). Furthermore, studies of preoperative staging accuracy that focus on patient selection for preoperative therapy are rare; therefore, whether EUS can reliably identify patients eligible for preoperative therapy (i.e., those with stage T2 or higher lesions) is still unclear. In addition, a recent meta-analysis demonstrated significant differences in the accuracy of EUS T staging between Western and Eastern reports. Sensitivities to distinguish T1 from T2 tumors were 71% in Western reports and 92% in Eastern reports (P<0.01), indicating that race or ethnicity may affect EUS accuracy (11). However, no prior studies have examined the role of race and ethnicity in particular in staging accuracy.
The main purpose of this retrospective study was to determine whether EUS accurately identifies patients with stage T2 or higher or node-positive disease who should be considered for preoperative therapy. We also sought to determine what variables, including race or ethnicity, are associated with the staging accuracy of EUS. Finally, we examined the accuracy of CT imaging for detecting lymph node metastasis and identified factors associated with it.
Methods
Patient selection
Having received Institutional Review Board approval, we conducted a retrospective review of a prospectively maintained database of the medical records of 8,260 patients with gastric or gastroesophageal adenocarcinoma treated at The University of Texas MD Anderson Cancer Center from 1995 to 2013. We identified patients who underwent gastrectomy after preoperative staging with EUS or CT. Patients with pathologic stage T0 tumors (no persistent cancer in the surgical specimen) were excluded from this study because the accuracy of pathologic staging after extensive biopsies or endoscopic mucosal resection is unknown. Because preoperative therapy affects both T and N staging (1,4), we excluded patients who had undergone such therapy. Patients’ demographic and clinicopathologic characteristics were collected. These characteristics included age, sex, race/ethnicity, smoking history, date of staging procedure (CT or EUS), date of surgery, tumor location, tumor size, presence of ulceration, presence of lymphovascular invasion, number of lymph nodes examined, histological grade (well/moderately or poorly differentiated adenocarcinoma), and presence of signet ring cell morphology.
Assessment of EUS T and N staging accuracy
T stage and N status as determined by preoperative EUS were compared with postoperative pathologic T and N staging to determine accuracy. T stage was defined according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual, and N status was defined as positive or negative. Before 2003, our institution’s EUS system used linear-probe endoscopes from Pentax Medical Co. (Tokyo, Japan). In 2003, we switched to linear-probe endoscopes from Olympus Optical Co. (Tokyo, Japan). Nodal status was determined by the endoscopists at the time of the EUS procedure based on the shape and size of the visualized lymph nodes. Fine-needle aspiration of the lymph nodes was not required to confirm the staging.
Assessment of CT N staging accuracy
To assess the accuracy of CT in detecting lymph node metastasis, we reviewed the included patients’ preoperative CT images. The images had to have been taken at MD Anderson Cancer Center, used intravenous contrast, and be of adequate quality. The original images were reviewed by a physician (N Ikoma) who was blinded to the EUS and surgical pathology results, tumor location, and other clinical information. Lymph nodes were considered positive when the short axis diameter was ≥6 mm on CT images. N status was defined as positive or negative.
Definition of staging accuracy
In general, patients with stage T2 or higher and/or node-positive tumors have significantly diminished survival rates (12,13). Based on the National Comprehensive Cancer Network (NCCN) guidelines and results from two large randomized trials (1,4) that have shown a survival benefit from adjunctive chemotherapy for such patients, our institution considers patients with clinical stage T2 or higher and/or node-positive tumors to be candidates for preoperative therapy (14). Therefore, for the purposes of this study, we defined the T-staging accuracy of EUS as its ability to distinguish early gastric cancer (stage T1a or T1b) from more advanced tumors (stage T2 or higher). Accuracy, sensitivity, and specificity were calculated based on this definition. For the purpose of comparing our results with previous reports, we also calculated the accuracy, sensitivity, and specificity of EUS for identifying summary T stage (T1–T4) and detailed T stage (T1a, T1b, T2–T4). Within the category of early T-stage tumors (T1 or T2), we also evaluated the ability of EUS to distinguish T1a tumors from T1b–T2 tumors by calculating accuracy, sensitivity, and specificity.
To examine factors associated with inaccurate EUS T staging, we defined overstaging as an EUS T stage that was higher than that indicated in the pathology report and understaging as an EUS T stage lower than that found in the pathology report, based on the 7th edition of the AJCC staging manual.
N status as determined by CT and EUS was compared to that indicated in the pathology report. Accuracy, sensitivity, and specificity for determining N status were calculated for both imaging modalities. EUS N status showed lower sensitivity than CT N status in this study, so we did not perform further analysis on EUS N status.
Statistical analysis
Univariate and multivariate analyses were performed to evaluate the association between clinicopathologic variables and inaccurate staging (overstaging or understaging of EUS T stage, false positive/false negative of EUS/CT N stage). The univariate analyses used the chi-square or Fisher exact tests as appropriate. The multivariate analyses used logistic regression models. Factors with a P value ≤0.25 in the univariate analysis were included in the logistic regression model, and the final models were determined using a stepwise method. Statistical analyses were performed using SAS software version 9.3 for Windows (SAS Institute, Cary, NC). All reported P values are 2-sided and were considered significant at the P<0.05 level.
Results
Patient characteristics
We identified 225 patients who had undergone gastrectomy without receiving preoperative treatment during the study period. We excluded patients who had no residual disease in the gastrectomy specimen (n=22), patients who had had a previous gastrectomy (n=8), and patients who did not undergo either CT or EUS at our institution within 3 months before surgery (n=10). We identified 187 patients who had undergone preoperative staging using EUS (n=145, 1995–2013) and/or CT (n=134, 2000–2013) prior to gastrectomy and whose images were adequate and available for review. Patient characteristics are detailed in Table 1. The patients were approximately 53% White, 19% Asian, 19% Hispanic, and 9% Black. Tumor location was most commonly in the antrum (approximately 47%). Approximately 47% of patients had early-stage (T1a/b) cancer, 44% had positive lymph nodes, 75% had tumors with poorly differentiated histology, and more than 50% had lymphovascular invasion.

Full table
Accuracy of EUS T stage
Detailed results of EUS T staging for each pathological T stage are shown in Table 2. The accuracy of EUS in identifying summary T stage (T1–T4) was 66% (96/145), and the accuracy of EUS in identifying detailed T stage (T1a, T1b, and T2–T4) was 52% (75/145). The accuracy, sensitivity, and specificity of EUS in distinguishing T1 from more advanced (T2–T4) tumors were 82%, 78%, and 85%, respectively. The accuracy, sensitivity, and specificity of EUS in distinguishing T1a from T1b–T2 tumors were 74%, 55%, and 86%, respectively.
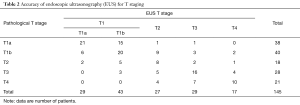
Full table
Variables associated with inaccurate diagnosis were investigated for 38 patients with EUS T-stage overstaging and 32 patients with understaging by comparing them to 75 patients with accurate EUS T staging. In the univariate analysis, tumor location (P=0.02) and lymphovascular invasion (P<0.01) were significantly associated with overstaging or understaging. Race/ethnicity approached a significant association with EUS accuracy (P=0.10). Histological grade (P=0.90), presence of signet ring cell histology (P=0.58), presence of ulceration (P=0.80), and the interval between EUS and surgery (<1, 1–2, or >2 months; P=0.74) were not associated with inaccurate staging. In the multivariate analysis, lymphovascular invasion [odds ratio (OR), 7.51; 95% confidence interval (CI), 1.91–29.50; P<0.01] and white race (OR, 3.75; 95% CI, 1.31–10.75; P=0.01) significantly predicted EUS T-stage understaging (Table 3).

Full table
Accuracy of EUS/CT N status
The accuracy, sensitivity, and specificity of N status were 65%, 49%, and 79% with CT and 66%, 29%, and 95% with EUS, respectively. Sensitivities and specificities of EUS and CT in determining N status for each T stage are shown in Table 4. In the univariate analysis, histological grade (P=0.01) and lymphovascular invasion (P=0.09) were associated with incorrect determination of N status on CT. Tumor size (P=0.21), location (P=0.33), patient race/ethnicity (P=0.62), presence of signet ring cell morphology (P=0.16), presence of ulceration (P=0.20), and interval between CT and surgery (P=0.21) were not significantly associated with inaccurate determination of N status on CT. In the multivariate analysis, lymphovascular invasion was associated with false-negative N status on CT (OR, 3.79; 95% CI, 1.34–10.73; P=0.01), and well- or moderately differentiated histology was associated with false-positive N status on CT (OR, 7.14; 95% CI, 2.00–25.44; P<0.01) (Table 5).
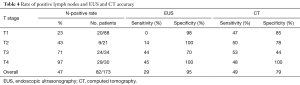
Full table

Full table
Discussion
In this study, we found that EUS T staging is reasonably accurate (82%) for distinguishing early-stage (T1) from more advanced (≥T2) gastric cancer. EUS and CT had unexpectedly low sensitivities (29% and 49%) and high specificities (95% and 79%) for determining N status. We also found that race can affect EUS T-staging accuracy. As expected, the presence of lymphovascular invasion was a risk factor for understaging T stage on EUS and for false-negative CT N-status findings. Our results indicate that EUS T stage can identify candidates for preoperative therapy (patients with stage T2 or higher tumors) with high accuracy.
Accurate preoperative staging is imperative for patient selection for preoperative therapy. The main difference of this study from previous ones is its focus on EUS’s ability to distinguish T1 from T2–T4 lesions. The vast majority of previous reports and review articles about EUS accuracy have evaluated its ability to distinguish T1–T2 from T3–T4 tumors (7,10,11,15), although no previous high-powered randomized studies of preoperative treatment for gastric cancer applied such criteria for patient selection (1,4). Previously published data showed that gastric cancer patients with stage T2 or higher tumors and/or node-positive disease benefit from perioperative chemotherapy (1,4). In the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial, which showed the benefit of perioperative chemotherapy in patients with gastric cancer, 32% of the study cohort had stage T2 tumors; patients with T2 lesions are likely to benefit from preoperative treatment (1). Reports showing lower survival rates in patients with T2 lesions than in those with T1 lesions, independent of lymph node status, further support the idea of providing pre- or post-operative treatment for patients with T2 lesions (12,16). Because we consider, in accordance with NCCN guidelines, that patients with stage T2 and higher tumors are likely to benefit from preoperative treatment, we aimed to assess the ability of EUS to distinguish T1 vs. T2–T4 lesions.
Reports of EUS T-staging accuracy have shown significant heterogeneity (7,8,10,11); therefore, special caution must be taken when using EUS to select patients for preoperative therapy. A recent Cochrane review reported that EUS had 85% sensitivity and 90% specificity in differentiating T1 from T2 tumors (11) and that better sensitivity was found in Eastern reports than Western reports (92% vs. 71%; P<0.01). We found three other studies reporting the accuracy of EUS from the United States after 2000 (17-19), and they uniformly reported that EUS T staging had low accuracy (41–57%). The results of our study show relatively higher accuracy than those studies did (accuracy for summary T stage of EUS was 66%), but not as high as that reported in studies from Asia. This prompted us to evaluate whether the racial or ethnic component of the patient cohorts could account for these discrepancies; we found that white race was associated with understaging of EUS on multivariate analysis. In addition, lymphovascular invasion, which is found in the majority of gastric cancer cases in the United States, was associated with understaging. More racially diverse patient populations and the higher frequency of lymphovascular invasion, reflecting the more aggressive morphology of gastric cancer in the West, may explain the discrepancies between the accuracies reported in Western and Asian studies.
Preoperative diagnosis of lymph node involvement in gastric cancer patients is challenging. Even intraoperative tactile assessment of nodes has low accuracy. Sano et al. (20) studied 522 patients with early-stage gastric cancer and reported 32% sensitivity and a false-positive rate of 69% for intraoperative assessment of lymph node involvement. Seto et al. (21) reported that intraoperative lymph node evaluation had lower sensitivity for determining N status in patients with undifferentiated tumors. These reports indicate that the size of the lymph nodes is not a reliable marker of lymph node involvement, especially in patients whose tumors have poorly differentiated histology. In a review article, Kwee et al. (22) reported a wide range of reported sensitivities (16.7–96.8%) and specificities (48.4–100%) for EUS and sensitivities (62.5–91.9%) and specificities (50.0–87.9%) for CT in evaluating lymph node status. The authors concluded that no imaging modality consistently detects lymph node involvement in gastric cancer, even in this era of advanced technology. The results of our study support these previous reports and confirm that neither EUS nor CT imaging N status can reliably be used for patient selection for preoperative therapy.
It remains unknown whether T1N1 tumors benefit from pre- or postoperative therapy. The current NCCN guidelines (version 3, 2016), based on the Adjuvant Capecitabine and Oxaliplatin for Gastric Cancer After D2 Gastrectomy (CLASSIC) trial (23), recommend adjuvant chemotherapy for all patients with node-positive tumors; however, only 1% of patients in the CLASSIC trial cohort had T1N1 disease. The Japan Clinical Oncology Group (JCOG) 8801 trial failed to demonstrate a benefit for adjuvant chemotherapy in serosa-negative gastric cancer patients (T1–T3); approximately one third of the patients in that cohort had T1 lesions (24). Considering the results of the JCOG 8801 trial, a subsequent trial in Japan, The Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer trial (3), excluded T1N+ patients and showed a benefit for adjuvant chemotherapy in patients with stages II/III gastric cancer. Future studies are warranted to determine the benefit of including N status in assessing eligibility for preoperative therapy, and such studies should include a multicenter trial in the West. In the meantime, the low accuracies of preoperative lymph node evaluation reported in this study and in previous Western studies, along with the questionable benefit of perioperative therapy in patients with T1N1 lesions, should be taken into account when considering patients for perioperative treatment.
The major limitation of this study is its retrospective nature, which carries an unavoidable risk of selection bias. Since the vast majority of patients with advanced gastric cancer at MD Anderson receive preoperative therapy, our sample of patients who did not receive such therapy was limited. The relatively long study period (18 years) is another limitation. To improve the validity of the study, only patients for whom high-quality CT images were available were included. As a result, the study population was smaller for CT imaging than for EUS. Differences in technical skills and the experience of the endoscopist may have affected the accuracy of EUS, but this is a limitation of all EUS studies, and the gastroenterologists who participated in this study all have had subspecialty training and experience in a high-volume practice focusing on gastric and gastroesophageal cancer.
In conclusion, EUS was highly accurate for differentiating stage T1 from stages T2–T4 gastric cancer, but both CT and EUS had low sensitivities and high specificities for determining N status. These accuracies and associated variables should be considered when selecting patients for preoperative therapy in gastric cancer. EUS T stage may better guide indications for preoperative therapy than clinical N stage on EUS or CT imaging. White race and presence of lymphovascular invasion were associated with T-stage understaging on EUS.
Acknowledgements
Amy Ninetto, PhD, Department of Scientific Publications, MD Anderson Cancer Center, for editorial assistance.
Funding: Supported in part by the NIH/NCI under award number P30CA016672 and used the Clinical Trials Support Resource.
Footnote
Conflicts of Interest: Presented at The American Society of Clinical Oncology Gastrointestinal Cancer Symposium, January 21, 2016.
Ethical Statement: The study was approved by Institutional Review Board, with protocol number of PA13-0168.
References
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531-46. [Crossref] [PubMed]
- Ajani JA, Barthel JS, Bekaii-Saab T, et al. Gastric cancer. J Natl Compr Canc Netw 2010;8:378-409. [Crossref] [PubMed]
- Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc 2011;73:1122-34. [Crossref] [PubMed]
- Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. Am J Gastroenterol 2008;103:1801-9. [Crossref] [PubMed]
- Cardoso R, Coburn N, Seevaratnam R, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer 2012;15 Suppl 1:S19-26. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol 2007;25:2107-16. [Crossref] [PubMed]
- Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015.CD009944. [PubMed]
- Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449-61. [Crossref] [PubMed]
- Sarela AI, Turnbull AD, Coit DG, et al. Accurate lymph node staging is of greater prognostic importance than subclassification of the T2 category for gastric adenocarcinoma. Ann Surg Oncol 2003;10:783-91. [Crossref] [PubMed]
- Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 2006;24:3953-8. [Crossref] [PubMed]
- Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001;49:534-9. [Crossref] [PubMed]
- Kunisaki C, Makino H, Kimura J, et al. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery 2010;147:204-11. [Crossref] [PubMed]
- Spolverato G, Ejaz A, Kim Y, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg 2015;220:48-56. [Crossref] [PubMed]
- Fairweather M, Jajoo K, Sainani N, et al. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol 2015;111:1016-20. [Crossref] [PubMed]
- Bentrem D, Gerdes H, Tang L, et al. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol 2007;14:1853-9. [Crossref] [PubMed]
- Sano T, Kobori O, Nagawa H, et al. The macroscopic diagnosis of lymph node metastasis from early gastric cancer. Surg Today 1994;24:37-9. [Crossref] [PubMed]
- Seto Y, Nagawa H, Muto T. Intra-operative diagnosis of N2 lymph node metastasis of gastric cancer. Hepatogastroenterology 1997;44:838-41. [PubMed]
- Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009;12:6-22. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Nakajima T, Nashimoto A, Kitamura M, et al. Adjuvant mitomycin and fluorouracil followed by oral uracil plus tegafur in serosa-negative gastric cancer: a randomised trial. Gastric Cancer Surgical Study Group. Lancet 1999;354:273-7. [Crossref] [PubMed]


