Heart applications of 4D flow
Introduction
Cardiac magnetic resonance (CMR) flow analysis has a wide range of established applications: for example, congenital heart disease and valves disease are currently studied with phase contrast (PC) imaging, which has proved to be accurate and reliable in this specific context (1), which have proved and demonstrated specifically with traditional PC imaging. Unfortunately, names for this technique vary a lot among researchers. In this review, we will refer to this technique as 4D flow imaging. This review covers the main MRI methods for measuring blood and myocardial motion, explaining the basic physic principles of PC imaging, which are the same as the 4D flow imaging. Phase-contrast MRI of blood flow is non-invasive, without ionizing radiation and can measure flow accurately 1 with flexibility of spatial and temporal resolution and has access to all directions of flow at any location in the body. It gives a quantification and graphic representation of different parameters of flow in a given vessel. The same PC sequences are used to collect data for phase-contrast angiography, which uses a different reconstruction method and produces 3D flow-enhanced images.
CMR imaging is difficult to perform on patients with life-support apparatus. Most CMR requires breath-hold co-operation for collecting raw data over several cycles. Consistent cardiac timing in each cycle is assumed and can be adversely affected by cardiac arrhythmia. Many systems have an arrhythmia rejection function. Some others have “retro-gated” techniques to correct abnormal cardiac intervals while scanning.
Blood flow has many parameters: e.g., the total volume of blood per cardiac cycle or per minute, stroke volume, the mean velocity over the vessel area, or averaged over the time of a cardiac cycle; the shape of the velocity distribution over the vessel area is known as flow profile, or over time through the cycle known as velocity waveform (again, the terms are not standardized), and the amount of turbulent flow, which is small in normal circulation.
Angio-MRI sequences
Moving structures can produce artifacts in MR images. Anyway, at the same time, they offer the possibility to obtain functional information by the effect that Radio-frequency pulses and field gradients have on protons’ spin during the image acquisition. Particularly three main procedures can be used to represent blood flow in vascular structures: (I) time of flight (TOF), which relies on the effect of a different time for reaching the steady state of longitudinal magnetization for still and moving structures. (II) PC considers the different effect of the reading gradient of gradient-echo sequences (which has a dephasing and a rephasing lobe of excited spins, and it is effective only if the molecules do not move during acquisition). (III) Contrast-enhanced magnetic resonance acquisition (CE-MRA) which, unlikely the former ones, needs the administration of a contrast medium with the subtraction of the acquired images before and after the administration itself.
PC sequences fundamental bases
The concept of PC and its quantification were described by Carr and Purcell in 1954 (2) who reported the first observation of coherent motion on MR, and by Hahn in 1960, who applied flow-sensitive MR to detect sea movements. Since then, two-dimensional and time-resolved PC MRI has become available on all modern MR systems and is an integral part of clinical protocols assessing cardiac and valve function in the heart and large vessels flows. These are Gradient-Echo sequences in which the reading gradient (aka frequency gradient, activated at last in a standard Gradient-Echo sequence) is activated in one direction, dephasing the rotation of spins, and then in an opposite direction, putting the spins back in-phase (Figure 1). This happens only to static tissues, whereas the opposite reading gradients could have a different intensity on every position of a spin of moving tissues, therefore not allowing a complete phase recovery. Usually bipolar reading gradients are applied on one anatomic axis only (x-, y-, or z-) to provide flow sensitivity exclusively in a specific direction. However, flow-encoding gradients may be applied along two or more axes at the same time to measure flow sensitivity along any arbitrary direction. The flow data obtained from a PC sequence study may be obtained in 1D, 2D, 3D, or 4D modes. PC MRI can be used as well to depict vessel anatomy in an angiographic way as it was described by Dumoulin et al. in 1989 (3).
One of the main disadvantages of PC compared to other pulse sequence is the acquisition time. To codify flow or movement along one axis (1D) two series of data must me confronted. To analyze 3 orthogonal directions four series of data must be acquired: one with movement compensation and three with flux analyzed along all the three axes. It is possible to estimate the different phase of moving fluids through the sequence of three different orientations of the reading gradient.
The following sequence of events is used in order to obtain PC images: firstly, images with bipolar gradients are obtained so that moving objects are evident; secondly, a sequence with gradients which can compensate flux are applied and a second set of images without flux component are obtained these two first sets of images have different velocity-dependent signal phase but otherwise identical sequence parameters. Finally, a subtraction between the first two acquisitions with phase information is performed so that unknown background phase is removed and only moving objects are evident. Calculation of the phase differences are obtained with the sequent (4):
|
∆Φ=γ∙∆m∙v
| [1] |
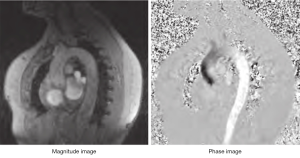
Generally, MR images are displayed so that brighter pixels contain more signal from a specific sequence; higher signal means more magnetization (M images) from a given pixel. If a pixel contains static tissue, the effects of the two opposite gradient pulses does not generate any image/signal, but if the tissue moves in the time between the pulses, the result is a phase shift, directly proportional to the velocity of the moving object (i.e., blood flow). This reconstruction method is used in PC images to obtain venograms or angiograms. The main problem about this reconstruction is that does not provide directionality or quantitative information. The magnetization measured in each pixel is a vector which has two main properties: size and direction. The direction of the vector rotates with a frequency which is compared by the machine to a reference rotating source (oscillator). This ahead or behind rotation is known as “phase shift”. In basic MRI sequences information given from the phases are analyzed to obtain information about the position of an atom (1). The phase is affected by uncontrolled factors like main field non-uniformity and chemical shift, so that the phase reconstruction images (P images, or velocity mapping images) are not normally displayed. In PC sequences both M images and P images are displayed in a dynamic mode (cine mode) giving information about the flow changes during a given time (generally a heart cycle) (Figure 2).
The entity of phase variation deriving from moving structures can change with the modification of the slope of the reading gradient.
The amplitude, spacing, and duration of the bipolar gradients determine the degree of sensitivity to different flows (arteriosus vs. venous). This is controlled by an operator-selectable parameter known as velocity encoding (VENC) which must be prearranged in any PC study so that the maximum velocity selected corresponds to a 180° phase shift in the data obtained. Proper setting of VENC is critical to the performance of the study. VENC is measured in cm\s and it should be used as the highest value of flow velocity that we are expecting in the target vessel.
|
VENC = π/[γA−gτ]
| [2] |
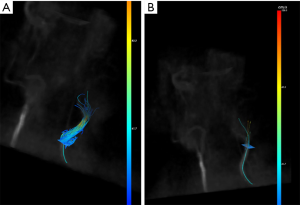
Where is the phase variation and the denominator reflects the properties of the bipolar gradient, such as duration, separation between lobes, etc. The VENC parameter is used to reconstruct P images: on the imaging domain the two datasets undergo Fourier transform to obtain a reference scan image and a velocity-encoded scan image which are then subtracted (Figure 3) with the calculation of the phase shift based on the succeeding equation.
Typically, the VENC can only be estimated since its best value is normally not known in advance. Frequently a study will be repeated using 2–3 different VENCs based on the expected velocities. If the selected VENC is set too high, the range of flows imaged will span only a limited phase shift range. The signal-to-noise of the image and quality of the data will suffer. Small velocity differences on flow studies will not be distinguishable, and vessels with slow flow may be impossible to see. It determines the highest and lowest detectable velocity encoded by a phase-contrast sequence. Therefore, VENC =100 cm/s describes a phase-contrast experiment with a measurable range of flow velocities of ±100 cm/s.
If VENC is chosen too low, velocity aliasing may occur with faster flows not being appropriately characterized. For example, if the predetermined VENC is 50 cm/s, the gradient is adjusted so that a flow of 0 and +50 cm/s are assigned a phase of +0° and +180° respectively. If the actual velocity is +75 cm/s, this flow will be mapped as +270°, a value that cannot be distinguished from a phase shift of −90°. The reconstruction will assign a flow of 25 cm/s in the opposite direction.
4D flow acquisition guidelines
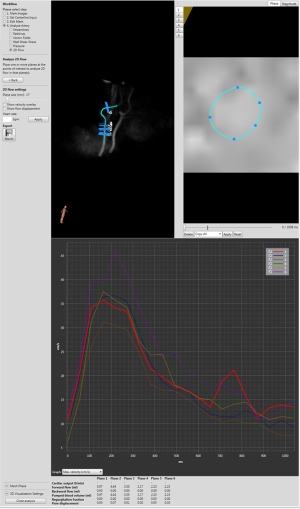
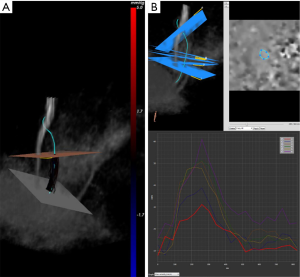
Three-dimensional spatial encoding combined with three-directional velocity-encoded PC MRI (aka 4D flow) has recently drawn increasing attention. Modern technical developments allow the comprehensive acquisition and analysis of flow dynamics with full volumetric coverage of the territory of interest. By doing so, a posteriori analyses of hemodynamics and flow analysis (as well as CSF) have become possible. These sequences can be depicted with an advanced post-processing analysis as three-dimensional images also in a “CINE” mode, for the visualization of blood flow, represented as vectors, streamlines and particles movement (Figure 4). It is also possible to estimate many valuable hemodynamic biomarkers such as wall shear forces, pulse wave velocity, pressure gradients and other measures.
Guidelines on how to acquire 4D flow sequences have been drawn in the last few years. Some of the most notably important are written on a paper of Markl et al. from 2012 (5) and on a paper from Dyverfeldt et al. from 2015 (6). In these papers they describe the state of the art of the technical acquisition (e.g., Cartesian and radial data acquisition), specify many acquisition parameters for different part of the body and show optimized parameters for different patient population such as children.
Errors and limitations
Phase-contrast techniques have been shown to be better than Doppler US in measuring mean flow. Doppler US assumes that there is a constant velocity over the whole vessel area during time. However, MR imaging can take into consideration the variation of flow in the vessel within the specified spatial resolution. Therefore, Doppler US tends to overestimate the mean flow in larger vessels (7) but it is more easily applicable in measuring peak velocities under various physiologic conditions (e.g., Valsava maneuver). The clinical relevance of these principal differences must be discussed with caution, as each technique gives different and still clinically useful results. However, knowledge of the technical limitations can help one find the most sensible result in the given situation.
The most common error that can be made in a PC or 4D flow study is an improper setting of the VENC. An encoding velocity set too high results in noise. On the opposite if VENC is set too low the result is aliasing. Noise disturbs the estimation of the peak velocity (Vmax) because it may be masked by noise peaks. Anyway, it has less influence on the assessment of the flow because the noise is averaged over several voxels. Consequently, larger deviations from the ideal VENC can be tolerated if the peak velocity is not the main interest of the measurement. Moreover, if the VENC is set too high, it has been reported an association with an underestimation of slow flows during diastole (8).
A deviation of imaging plan could affect the measurements of flow. The best precision is obtained when the imaging plane is positioned orthogonal to the main direction of flow. This is more easily achieved in 4D flow imaging in which vectors of flow are displayable and the plane can be easily oriented retrospectively in the three-main axis. A maximum deviation from the orthogonal plane is ±15° (4).
The temporal resolution is another important parameter of any PC based sequence. Each of these sequences is heart-gated and so each image has a set of frames at a different point of a cardiac cycle. For most situations, a value of 30 frames is enough, representing a good compromise between time for postprocessing and the temporal accuracy of the flow measurement. When a low temporal resolution is used, the peak velocity and flow in the great arteries might be underestimated.
Finally, it must be bear in mind that partial volume effects are present where an inadequate spatial resolution is present. The pixel size must not exceed on third of the vessel diameter (9). With decreasing spatial resolution, partial volume effects cause underestimation of the flow and peak velocity.
Guidelines for data analysis
A typical 4D flow exam includes about 4,000–8,000 raw magnitude and velocity component images, depending on the spatial and temporal resolution and anatomical extent.
Special software is required to extract the information from the velocity images either in 4D flow or in basic PC sequences. This software can usually be obtained from the vendors of the MR imaging unit in regards of basic PC but most vendors have not implemented yet a 4D flow independent analysis software: a third-part software should be used, such as CAAS (PIE Medical Imaging, Maastricht, The Nederland) GTFlow (Gyrotools, Zurich, Switzerland), EnSight (CEI Inc., Apex, NC) or Arterys (San Francisco, CA USA) which is FDA approved. One really important thing is to define contours in the magnitude images and always cross-check them in the velocity images. Partial volume effects or large movements of the vessel may result in inadequate contour definition in the magnitude images. This problem may be easily corrected by comparing magnitude with the velocity images. Some experiments have shown that the velocity images can be more reliable for estimation of vessel boundaries than the magnitude images (10). For the estimation of the peak velocity, a small ROI within the vessel is appropriate. Indeed, it may give improved results than a ROI encompassing the entire vessel, since arbitrary phase values in the tissue next to the vessel are avoided (11).
The velocity data can also be visualized as a vector field in a single plane, as well as using streamlines or particles traces. By selecting a plane of interest, the velocities at the level of that specific plane can be represented as individual vectors (Figure 4). It is suitable for an analysis of local flow patterns, such as vortex formation (12) or helix direction (13).
Particle traces are path-lines that an imaginary particle would take over vessel branches (14). Imaginary particles arising from a specific plane of interest, can be released in a desired heart phase and can be followed through the cardiac cycle representing the very pathways that blood flow follows.
One of the main advantages of 4D flow imaging compared to traditional PC sequences is the retrospective quantification of blood flow. During the MRI review for report, it is common to suspect new diagnoses, which were not previously described or thought of during acquisition. Hence, the standard 2D PC-MRI sequences may have not been performed during the scan, resulting in a need for the patient to be rebooked for an additional scan. 4D flow allows post-scan planes selection without being limited by the pre-acquired 2D PC-MRI: unlike 2D PC-MRI, all the relevant flow information is stored within the 4D flow volume as a backup.
The software can calculate the pressure difference (P) between a reference and an obstruction plane (placed at site of obstruction/stenosis). Pressure difference measurements can be performed to asses a stenosis. When a stenosis is present, a pressure drop is expected at the location of the stenosis (obstruction plane) compared to a healthy part of the vessel (reference plane) (15). This calculation is based on the modified Bernoulli equation:
[3]
Where ρ is the mass density of blood, νreference and νobstruction are the velocities in the reference plane and the obstruction plane, respectively.
4D flow provides the possibility to visualize wall shear stress (WSS) in a 3D segmented model of the vessel of interest. WSS analysis is still subject of ongoing research and not used for clinical decision support. Wall shear inside blood vessels arises from the blood flow that shears against the vessel wall. Only the blood flow parallel to the vessel wall (V parallel) contributes to the shear against the wall. Wall shear can be quantified by means of the wall shear rate (© or WSR). The WSR is given by the gradient of the velocity profile of the parallel blood flow at the vessel wall (16). The equation for the WSR in [s–1] is:
[4]
where r is the radial distance perpendicular to the vessel wall. For correct WSS calculation the point of the wall must be found. Software do this by extrapolating the wall to the location where the velocity is zero. The wall location is automatically determined and calculated independently of the mesh. The vessel wall is calculated according to similar methods described in literature (17,18). WSS is then calculated by multiplying the WSR with the viscosity of blood. Based on the 3D reconstruction of the vessel a visualization of WSS distribution is shown. Note that multiple parameters related to image acquisition influence accuracy of the WSS calculation, which could lead to an underestimation of WSS values (19).
4D flow is also used for studying energetics in the blood low. The kinetic energy (KE) of a moving particle with a mass m (particle volume multiplied by the blood density) and velocity v, can be calculated with the formula ½mv2. The KE at a specific time point can then be calculated by summing the KE of each voxel within a specified anatomical region.
Clinical applications
In the last years, many literature papers have been published on the utilization of 4D flow CMR for examining either the normal flow patterns in cardiac chambers or their alterations in congenital and acquired pathology. The next sections are divided according to different anatomical sections to focus on the latest advancement on flow alterations on each different section of the heart and great vessels.
Atria
The physiological flow of blood through the left atrium consist in a specific path from the pulmonary veins to the mitral valve. Through this path there is a para-physiological formation of vortices, demonstrated with a CMR study using 4D flow sequences (20). In the right atrium blood flows from inferior and superior vena cava and turns anterior after entering the atrium. Assessment of atrial flow patterns and blood flow velocity is important in patients with atrial fibrillation for its association with an increased risk of embolic stroke due to thrombus formation in the left atrium (21). Patients with atrial fibrillation show global and regional changes in atrial dynamics, such as decreased blood flow velocities()in this contest, 4D flow analysis could be helpful in evaluating the risk of thrombosis and guiding the anticoagulant therapy) which could be a helpful indicator in risk assessment for thrombogenesis in these patients and thus could benefit of further studies to see how this technique could affect therapy such as oral anticoagulant (22,23). From a physiological point of view 4D flow has been used to expand our knowledge on normal heart cycle, giving an important insight of the kinetic energy (KE) and its contribution to blood acceleration (24). Cardiac magnetic resonance (CMR) with 4D flow is the only imaging modality that allows for direct quantification of KE in a volume of blood. In patients with mitral regurgitation, highly disturbed flow patterns in the left atrium with elevated values of TKE develop, related to the severity of regurgitation (25). It has been proved that the amount and grade of mitral valve regurgitation can accurately be assessed with the use of 4D flow CMR with retrospective valve tracking (26).
Ventricles
Right ventricular function is a powerful prognostic indicator in many forms of disease, but its assessment remains challenging. RV dysfunction may alter the normal patterns of RV blood flow, but those patterns have been, until few years ago, incompletely characterized with traditional methods. A study on healthy subject with 4D flow sequences has characterized the natural flow through the right ventricle (27). The flow path from the inflow to the outflow tract of the right ventricle follows a curvature through the basal and mid-ventricular parts of the chamber, reflecting the heart anatomy. In contrast, the left ventricle inflow and outflow directions are nearly opposite in direction, with an acute-angle turning point in the midventricular and apical regions. The right ventricle can be affected by right-sided pathological conditions, like pulmonary hypertension, congenital heart disease, or secondarily involved in states with left ventricular dysfunction. Right heart is well assessed with echocardiography due to its position closer to the chest wall than the left ventricle, and for some pathology echocardiography is the gold standard Fredriksson et al. have recently demonstrated that 4D flow, compared to conventional MRI and echocardiography, can better detect subtle impairment of the right ventricle caused by primary left ventricle pathology (28). An advantage of right ventricle 4D flow measurements is that it evaluates not only the performance of the free wall and the interventricular septum but also the configuration of the entire ventricle and its interaction with the flow.
Pulmonary arteries
Pulmonary hypertension is defined as an increase in mean pulmonary arterial (mPAP) pressure greater than 25 mmHg at rest measured with invasive right heart catheterization. So far, the right heart catheterization has been the only method to identify with certainty the presence of a pulmonary hypertension. 4D flow can visualize abnormal vortical flow presence in the main pulmonary arteries of patients with pulmonary hypertension, with its possibility to depict streamlines. Different authors have focused on different flow signs visible with a 4D flow analysis. The first sign that has been demonstrated is the presence of vortices in the main pulmonary artery which correlates with the presence of elevated mean pulmonary arterial pressure invasively measured (29). Another study from Lungu et al. has mathematically proved a relationship between the flow Q though the mean pulmonary artery and pressure p with a 2D PC acquisition, which could be easily, and with more completeness achieved with a 4D acquisition (30). More studies must be performed on this topic, but Lungu’s group proved an accuracy of 92% diagnosing pulmonary hypertension, reducing the need for right heart catheterization in patients with suspected PH.
Aorta
The 3D flow, time resolved, CMR can provide good accuracy for the assessment of valve abnormalities through all four heart valves within a single acquisition (26). It has been proved that 4D flow imaging correlates well with echocardiography in detecting clinically relevant aortic regurgitation with a statistical agreement in quantification of the regurgitation itself and with the grading of the disease (31). An alteration of the aortic valve (e.g., bicuspid aortic valve), can affect the hemodynamics of the downstream aorta, altering the distribution of aortic blood flow helicity (32). Perhaps the most game-changing discovery revealed by 4D flow in the aorta is surprisingly related to stroke. It is well known that plaques of the ascending aorta and aortic arch are a cause of embolic stroke (33). However, compared to the ascending aorta, the incidence of complex aortic plaques is highest in the descending aorta, but these plaques are not considered an embolic source of stroke, unless a severe aortic valve insufficiency causes retrograde flow in case of plaque rupture (34). 4D flow has though revealed that retrograde embolization from complex descending aortic plaques is a likely event in patients with atherosclerosis and could reach all supra-aortic arteries even in patients without aortic valve insufficiency, indicating that descending aortic plaques should be considered a new embolic source of stroke, even in the absence of aortic valve insufficiency (35). This mechanism has been proved to be the only probable source of cerebral infarction in patients with unknown stroke etiology.
Conclusions
In this review, we have covered the basics of PC imaging, and its modern evolution, four-dimensional flow imaging, particularly with special attention on the heart applications. As we have seen, the most prominent implementations of this technique are still used for research studies. These studies have demonstrated how its application on clinical settings could be important in the diagnosis of congenital and acquired heart diseases. Shorter acquisition times have made 4D flow more widespread in clinical settings than in the past, still, further implementations are necessary to improve spatial and temporal resolution, and to give to 4D flow the strength necessary to qualitatively analyze flow in small vessels like coronaries, which could be a game-changing for the approach on coronary artery diseases. Furthermore, additional studies are needed to check the feasibility of using the flow hemodynamic information given by 4D flow analysis to stratify risk, follow up diseases and check the response to therapy, with regards of flow parameters changes after surgical reparation of congenital and acquired diseases. One of the main difficulties is the visualization of the data and assessment of values. The visualization is still limited by the scarce diffusion of post-processing software which is expensive, and which must be scientifically validated in clinical settings in all the different pathologies which could be studied with 4D flow. The lack of automatism for post-processing this huge amount of data is a limitation for the clinical use, since a single anatomical field analysis can take up to one hour. The assessment of standard values of reference is a challenge of the next few years, since they could be used as important markers of diseases.
On the other hand, as we have seen through this review, 4D flow allows us to unravel basic blood flow patterns in the heart in vivo, changing the way physiology is understood, and giving us additional bases to understand diseases and the changes they evoke in blood flow.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Luca Saba) for the series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.02.08). The series “Advanced Imaging in The Diagnosis of Cardiovascular Diseases” was commissioned by the editorial office without any funding or sponsorship. LS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from July 2019 to June 2021. FC serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from July 2019 to June 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gatehouse PD, Keegan J, Crowe LA, et al. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol 2005;15:2172-84. [Crossref] [PubMed]
- Carr HY, Purcell EM. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev 1954;94:30-8. [Crossref]
- Dumoulin CL, Hart HR Jr. Magnetic resonance angiography. Radiology 1986;161:717-20. [Crossref] [PubMed]
- Lotz J, Meier C, Leppert A, et al. Cardiovascular Flow Measurement with Phase-Contrast MR Imaging: Basic Facts and Implementation. Radiographics 2002;22:651-71. [Crossref] [PubMed]
- Markl M, Frydrychowicz A, Kozerke S, et al. 4D flow MRI. J Magn Reson Imaging 2012;36:1015-36. [Crossref] [PubMed]
- Dyverfeldt P, Bissell M, Barker AJ, et al. 4D low cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson. 2015;17:72. [Crossref] [PubMed]
- Evans AJ, Iwai F, Grist TA, et al. Magnetic resonance imaging of blood flow with a phase subtraction technique. Invest Radiol 1993;28:109-15. [Crossref] [PubMed]
- Buonocore MH. Blood flow measurement using variable velocity encoding in the RR interval. Magn Reson Med 1993;29:790-5. [Crossref] [PubMed]
- Tang C, Blatter DD, Parker DL. Accuracy of phase contrast flow measurements in the presence of partial-volume effects. J Magn Reson Imaging 1993;3:377-85. [Crossref] [PubMed]
- Kozerke S, Botnar R, Oyre S, et al. Automatic vessel segmentation using active contours in cine phase contrast flow measurements. J Magn Reson Imaging 1999;10:41-51. [Crossref] [PubMed]
- Hamilton CA, Moran PR, Santago P 2nd, et al. Effects of intravoxel velocity distributions on the accuracy of the phase mapping method in phase-contrast MR angiography. J Magn Reson Imaging 1994;4:752-5. [Crossref] [PubMed]
- Morbiducci U, Ponzini R, Rizzo G, et al. In vivo quantification of helical blood flow in human aorta by time-resolved three-dimensional cine phase contrast magnetic resonance imaging. Ann Biomed Eng 2009;37:516-31. [Crossref] [PubMed]
- Unterhinninghofen R, Ley S, Ley-Zaporozhan J, et al. Concepts for visualization of multidirectional phasecontrast MRI of the heart and large thoracic vessels. Acad Radiol 2008;15:361-9. [Crossref] [PubMed]
- Wigström L, Ebbers T, Fyrenius A, et al. Particle trace visualization of intracardiac flow using time-resolved 3D phase contrast MRI. Magn Reson Med 1999;41:793-9. [Crossref] [PubMed]
- Rengier F, Delles M, Eichhorn J, et al. Noninvasive pressure difference mapping derived from 4D flow MRI in patients with unrepaired and repaired aortic coarctation. Cardiovasc Diagn Ther 2014;4:97-103. [PubMed]
- Sui B, Gao P, Lin Y, et al. Noninvasive determination of spatial distribution and temporal gradient of wall shear stress at common carotid artery. J Biomech 2008;41:3024-30. [Crossref] [PubMed]
- Potters WV, van Ooij P, Marquering H, et al. Volumetric Arterial Wall Shear Stress Calculation Based on Cine Phase Contrast MRI. J Magn Reson Imaging 2015;41:505-16. [Crossref] [PubMed]
- Stalder AF, Russe MF, Frydrychowicz A, et al. Quantitative 2D and 3D Phase Contrast MRI: Optimized Analysis of Blood Flow and Vessel Wall Parameters. Magn Reson Med 2008;60:1218-31. [Crossref] [PubMed]
- Petersson S, Dyverfeldt P, Ebbers T. Assessment of the Accuracy of MRI Wall Shear Stress Estimation Using Numerical Simulations. J Magn Reson Imaging 2012;36:128-38. [Crossref] [PubMed]
- Fyrenius A, Wigstrom L, Ebbers T, et al. Three dimensional flow in the human left atrium. Heart 2001;86:448-55. [Crossref] [PubMed]
- Bernhardt P, Schmidt H, Hammerstingl C, et al. Patients With Atrial Fibrillation and Dense Spontaneous Echo Contrast at High Risk. J Am Coll Cardiol 2005;45:1807-12. [Crossref] [PubMed]
- Lee DC, Markl M, Ng J, et al. Three-dimensional left atrial blood flow characteristics in patients with atrial fibrillation assessed by 4D flow CMR. Eur Heart J Cardiovasc Imaging 2016;17:1259-68. [Crossref] [PubMed]
- Markl M, Carr M, Ng J, et al. Assessment of left and right atrial 3D hemodynamics in patients with atrial fibrillation: a 4D flow MRI study. Int J Cardiovasc Imaging 2016;32:807-15. [Crossref] [PubMed]
- Arvidsson PM, Töger J, Heiberg E, et al. Quantification of left and right atrial kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. J Appl Physiol (1985) 2013;114:1472-81. [Crossref] [PubMed]
- Dyverfeldt P, Kvitting JP, Carlhäll CJ, et al. Hemodynamic aspects of mitral regurgitation assessed by generalized phase-contrast MRI. J Magn Reson Imaging 2011;33:582-8. [Crossref] [PubMed]
- Roes SD, Hammer S, van der Geest RJ, et al. Flow assessment through four heart valves simultaneously using 3-dimensional 3-directional velocity-encoded magnetic resonance imaging with retrospective valve tracking in healthy volunteers and patients with valvular regurgitation. Invest Radiol 2009;44:669-75. [Crossref] [PubMed]
- Fredriksson AG, Zajac J, Eriksson J, et al. 4-D blood flow in the human right ventricle. Am J Physiol Heart Circ Physiol 2011;301:H2344-50. [Crossref] [PubMed]
- Fredriksson AG, Svalbring E, Eriksson J, et al. 4D flow MRI can detect subtle right ventricular dysfunction in primary left ventricular disease. J Magn Reson Imaging 2016;43:558-65. [Crossref] [PubMed]
- Reiter G, Reiter U, Kovacs G, et al. Magnetic Resonance-Derived 3-Dimensional Blood Flow Patterns in the Main Pulmonary Artery as a Marker of Pulmonary Hypertension and a Measure of Elevated Mean Pulmonary Arterial Pressure. Circ Cardiovasc Imaging 2008;1:23-30. [Crossref] [PubMed]
- Lungu A, Swift AJ, Capener D, et al. Diagnosis of pulmonary hypertension from magnetic resonance imaging–based computational models and decision tree analysis. Pulm Circ 2016;6:181-90. [Crossref] [PubMed]
- Chelu RG, van den Bosch AE, van Kranenburg M, et al. Qualitative grading of aortic regurgitation: a pilot study comparing CMR 4D flow and echocardiography. Int J Cardiovasc Imaging 2016;32:301-7. [Crossref] [PubMed]
- Lorenz R, Bock J, Barker AJ, et al. 4D flow magnetic resonance imaging in bicuspid aortic valve disease demonstrates altered distribution of aortic blood flow helicity. Magn Reson Med 2014;71:1542-53. [Crossref] [PubMed]
- Sen S, Hinderliter A, Sen PK, et al. Aortic arch atheroma progression and recurrent vascular events in patients with stroke or transient ischemic attack. Circulation 2007;116:928-35. [Crossref] [PubMed]
- Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation 2006;114:63-75. [Crossref] [PubMed]
- Harloff A, Simon J BS. Complex plaques in the proximal descending aorta: An underestimated embolic source of stroke. Stroke 2010;41:1145-50. [Crossref] [PubMed]