Pancreaticoduodenectomy with en bloc vein resection for locally advanced pancreatic cancer: a case series without venous reconstruction
Introduction
Pancreatic ductal adenocarcinoma is a formidable challenge as the fourth leading cancer cause of death in America with a 5-year survival of only 8% (1). This statistic number includes the over half of patients who are diagnosed with cancer metastasis with a 5-year survival of only 3%. However, for around 20% patients with localized pancreatic cancer, the postoperative outcome is relatively more optimistic with a 29% 5-year survival (1). Resection with clean margin (R0 resection) is associated with better survival. With recent advances in surgery, as well as adjuvant and neoadjuvant therapies, the outcome for patients with pancreatic cancer is improving. In the last decade, there have been advancements in the criteria for resectability, with a shift toward more aggressive resection to achieve clean margin. The concept of borderline resectable disease and the benefit of resecting pancreatic cancer involving the superior mesenteric vein (SMV) or portal vein (PV) has been introduced as safe procedure since the 1990s (2-4).
Currently, pancreatic cancer with SMV and/or PV involvement more than 180 degrees are considered borderline resectable if the portal venous flow can be restored. If the vessels are not reconstructable after resection of pancreatic cancer, we consider this cancer as locally advanced. Locally advanced pancreatic cancer comprises approximately 30% of pancreatic cancer patients and has a 5-year survival rate of approximately 6% (5). Resection of the PV and/or SMV without reconstruction is scarcely discussed in the literature (6). We presented our experience of a very unique series of cases in which venous reconstruction after resection was safely omitted. These cases emphasize the need for multi-disciplinary care and careful selection of patients with locally advanced pancreatic cancer for surgical resection at a high-volume center.
Current guidelines: borderline resectable vs. locally advanced pancreatic cancer
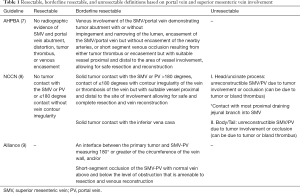
Consensus guidelines have been published by several organizations defining resectable, borderline resectable, and locally advanced pancreatic cancer based on its relationship with surrounding structures (Table 1) (7-9). The key surrounding structures are the celiac axis, superior mesenteric artery (SMA), common hepatic artery, and the SMV and/or PV.
Full table
With regard to the SMV/PV, all guidelines define borderline resectable disease with a reconstructable option after venous resection, and locally advanced disease with non-reconstructable venous occlusion. The International Study Group of Pancreatic Surgery (ISGPS) consensus statement by Bockhorn et al. describes a classification system of four types of venous resections for extrahepatic mesenteric-portal venous resection (10).
- Partial venous excision with venorrhaphy by suture closure;
- Partial venous excision using a patch venoplasty closure;
- Segmental venous resection with primary venovenous anastomosis;
- Segmental venous resection with interposed venous conduit and at least 2 anastomoses.
Notably, venous resection without reconstruction was not mentioned in these guidelines.
Ability of CT to predict need for venous resection
Determining resectability prior to surgery is dependent on high quality preoperative imaging and surgeon’s experience. Multidetector computed tomography (CT) is the most commonly used modality to assess vascular involvement and distant metastasis. The accuracy of CT to reveal resectability of pancreatic cancer was 76.9% (11). Multidetector CT protocols for pancreatic imaging typically include multiphasic thin-section imaging and multi-planar reconstruction (12). MRI is likewise an excellent modality for pancreatic cancer imaging, with equivalent performance for assessment of vascular invasion (12,13).
Ishikawa et al. described a classification system of tumor involvement with SMV/PV based on preoperative imaging (14). Based on SMV/PV involvement on preoperative imaging, the likelihood of vascular invasion can be estimated. For example, when the tumor-vessel involvement is over 180 degrees of the vessel, the need for vascular resection can be anticipated with 84% sensitivity and 98% specificity (15). In a 2012 study by Springett et al., the probability of vascular invasion was up to 40%, 80%, and 100% when the tumor has ≤180 degrees of contact, >180 degrees of contact, and 360 degrees of contact (16). Overall, the ability of CT to predict if vein resection is necessary in all cases is approximately 40% (17), and can be increasingly difficult after neoadjuvant therapy (18).
Overall survival and outcomes with venous resection
Mesenteric venous resection with reconstruction has been an accepted practice for pancreatic cancer with involvement of the SMV or PV since the 1990s when studies demonstrated equivalent survival of patients undergoing margin-negative venous resection compared to patients undergoing standard pancreaticoduodenectomy, as well as superiority of resection compared to nonsurgical treatment (3,4). A study comparing 75 patients with and without vein resection demonstrated no difference in survival between the two groups (19). More recent study by Roch et al. evaluating 90 patients with vein resection demonstrated that vein resection was not associated with worse overall survival at 1, 3, or 5 years (20). Other recent studies have also demonstrated patients with PV/SMV resection had equivalent survival outcome (21-25). Some studies have reported increased morbidity associated with venous reconstruction, providing caution to those considering this technique (26,27). A recent meta-analysis comparing venous resection with Whipple to palliative bypass and chemotherapy demonstrated no patients in the palliative group were alive at 2 years, while 40% of the resection group were alive at 3 years (28). Therefore venous resection, when R0 resection can be reasonably expected, has been accepted as the standard of care for indicated patients (29).
Patency rates
While venous resection without reconstruction is a novel concept in the literature, there are numerous studies evaluating postoperative stenosis or thrombosis of venous anastomoses. In 2015, Fujii et al. evaluated 197 patients who had undergone venous resection and reconstruction. Of these patients, three required immediate reoperation or anticoagulation for acute anastomotic thrombosis after surgery. Excluding these patients, the degree of patency at one year was more than 80% in 72 patients (30). Furthermore, Krepline et al. demonstrated that 4 of 41 venous resection patients (10%) were occluded after 12 months (31). In the shorter term, Javed et al. revealed that concentric narrowing was observed in 57.1% of patients, eccentric narrowing in 27.1%, complete or partial venous thrombosis in 10% at approximately 2 weeks after surgery (32). This occlusion and narrowing without significant morbidity are likely due to the development of collateral venous flow. If collateral veins are present prior to the operation, and may be preserved, a reconstruction of SMV/PV may not be necessary. Here we reported a series of 5 patients of this unique circumstance when vein reconstruction was not necessary after vein resection.
Our experience
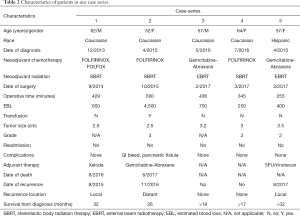
All patients were thoroughly evaluated by our multidisciplinary team prior to the decision to go to the operating room. They underwent a classic pancreaticoduodenectomy with portal lymphadenectomy, with meticulous attention to hemostasis and preservation of collateral venous flow. A brief summary of all patients may be seen in Table 2.
Full table
Patient example
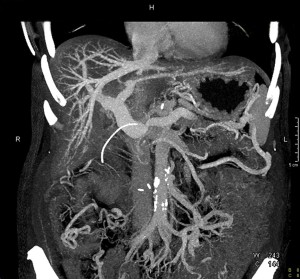
Patient 2 (Table 2) is a 52-year-old Caucasian female who was initially diagnosed with locally advanced pancreatic adenocarcinoma in April 2015. Initial CT imaging demonstrated long segment encasement of the SMV by cancer, as well as SMA abutment. She underwent 4 cycles of neoadjuvant FOLFIRINOX, as well as SBRT. Repeat CT after neoadjuvant therapy demonstrated slightly decreased size of the cancer with stable encasing and narrowing of the SMV below the PV/SV (splenic vein) confluence. Multiple collateral vessels were present on imaging (Figure 1).
She underwent operative exploration in October 2015. Due to the large tumor inside the head of the pancreas encasing the entire SMV, the distal pancreas was mobilized first. The SV and the PV were isolated with vessel loops. Pancreas was divided above the PV at the PV/SV confluence. The SMA was carefully dissected out on the left side of the PV. Due to the long segment of SMV encasement below the PV/SV confluence, we decided to divide the vein above the tumor and resect the Whipple specimen en bloc with SMV. After resection, a 4-cm gap was present between the proximal PV/SV confluence and the 3 branches of the SMV. We carefully preserved the collateral veins in the mesocolon. Her small bowel and colon were all viable and without congestion. To repair the narrowed PV/SV confluence after the resection, we performed a patch repair using a piece of cryopreserved femoral vein. The SMV was left in discontinuity.
Pathology returned as pT2N1M0, 2.5 cm poorly differentiated adenocarcinoma of the head of the pancreas, 3 of 17 lymph nodes positive, negative margins, and a grade 3 (poor/no response) response to therapy. The patient was treated with adjuvant Gemcitabine and Abraxane. She had distant recurrence in November 2016 and deceased in June 2017, approximately 20 months after surgery (26 months after diagnosis).
Considerations for case selection
Our series demonstrated the feasibility of the pancreaticoduodenectomy with vein resection without reconstruction for a group of patients with locally advanced pancreatic cancer. These patients overall did well following their surgeries, with 2 of 5 patients living 20–24 months after surgery (26–32 months from diagnosis) and the remaining 3 patients currently alive with ranges of 5–10 months since surgery (17–32 months from diagnosis).
It is prudent to emphasize the rigorous preoperative multidisciplinary evaluation by our experts prior to proceeding to the operating room. As previously described, while it is feasible to resect pancreatic cancer with venous involvement to achieve a R0 status, this surgical resection is not without risk (3,4,19-27). Patients in our study all had extensive venous collateralization that had developed before surgery. This collateral venous flow poses the challenge for surgical resection. Careful attention to hemostasis is imperative. Preservation of left sided collateral veins and mesenteric collateral veins is key to maintain adequate mesenteric venous drainage when the occluded SMV will be en bloc resected during pancreatectomy. At our institution, all pancreatic cancer patients undergo high-quality cross-sectional imaging with intravenous contrast appropriately timed to assess the portomesenteric axis and collateralization.
Patients with borderline or locally advanced pancreatic cancer will receive appropriate neoadjuvant treatment based on their performance status in the setting of clinical trials. Patients with local disease progression or metastasis, cavernous transformation of the porta hepatis will not be candidates for surgery.
Surgeons have to evaluate the patterns of venous collateralization before surgery. For planned pancreaticoduodenectomy, patent left-sided collateral venous flow through a PV/SV confluence or a dilated left gastric vein is necessary for possible successful resection. Alternatively, if distal pancreatectomy is planned, patent right-sided collateral venous flow through the pancreatic head, omentum and mesocolon to the liver is necessary. These patterns are critical for evaluation of any cases with venous involvement, as previous studies have shown that the ability to predict venous resection can be challenging (17,18). Knowing all options prior to entry in the operating room empowers the surgeons with the greatest likelihood of achieving R0 resection.
Conclusions
Our current series demonstrated the feasibility of using vein resection without reconstruction during pancreatectomy for pancreatic cancer. This helped us to achieve the R0 resection and potential long-term survival. With improvements in systemic and loco-regional therapies, we will have an increased rate of surgical resection in patients with borderline resectable or locally advanced pancreatic cancer (31). It is critical to continue rigorous preoperative multidisciplinary evaluation and selecting appropriate patients for this surgical resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Allema JH, Reinders ME, van Gulik TM, et al. Portal Vein Resection in Patients Undergoing Pancreatoduodecetomy for Carcinoma of the Pancreatic Head. Br J Surg 1994;81:1642-6. [Crossref] [PubMed]
- Fuhrman GM, Leach SD, Staley CA, et al. Rationale for En Bloc Vein Resection in the Treatment of Pancreatic Adenocarcinoma Adherent to the Superior Mesenteric-Portal Vein Confluence. Pancreatic Tumor Study Group. Ann Surg 1996;223:154-62. [Crossref] [PubMed]
- Gupta R, Amanam I, Chung V. Current and Future Therapies for Advanced Pancreatic Cancer. J Surg Oncol 2017;116:25-34. [Crossref] [PubMed]
- Maley WR, Yeo CJ. Vascular Resections During the Whipple Procedure. Adv Surg 2017;51:41-63. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Ed 2017. Available online: (last accessed December 2017).http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787-95. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Valls C, Andía E, Sanchez A, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 2002;178:821-6. [Crossref] [PubMed]
- Zaky AM, Wolfgang CL, Weiss MJ, et al. Tumor-Vessel Relationships in Pancreatic Ductal Adenocarcinoma at Multidetector CT: Different Classification Systems and Their Influence on Treatment Planning. Radiographics 2017;37:93-112. [Crossref] [PubMed]
- Treadwell JR, Zafar HM, Mitchell MD, et al. Imaging tests for the diagnosis and staging of pancreatic adenocarcinoma: a meta-analysis. Pancreas 2016;45:789-95. [Crossref] [PubMed]
- Ishikawa O, Ohigashi H, Imaoka S, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg 1992;215:231-6. [Crossref] [PubMed]
- Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 1997;168:1439-43. [Crossref] [PubMed]
- Springett GM, Hoffe SE. Borderline resectable pancreatic cancer: on the edge of survival. Cancer Control 2008;15:295-307. [Crossref] [PubMed]
- Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg 2014;18:269-78. [Crossref] [PubMed]
- Cassinotto C, Cortade J, Belleannée G, et al. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol 2013;82:589-93. [Crossref] [PubMed]
- Leach SD, Lee JE, Charnsangavej C, et al. Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg 1998;85:611-7. [Crossref] [PubMed]
- Roch AM, House MG, Cioffi J, et al. Significance of Portal Vein Invasion and Extent of Invasion in Patients Undergoing Pancreatoduodenectomy for Pancreatic Adenocarcinoma. J Gastrointest Surg 2016;20:479-87; discussion 487. adenocarcinoma. [Crossref] [PubMed]
- Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935-49; discussion 949-50. [Crossref] [PubMed]
- Ravikumar R, Sabin C, Abu Hilal M, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014;218:401-11. [Crossref] [PubMed]
- Kulemann B, Hoeppner J, Wittel U, et al. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg 2015;19:438-44. [Crossref] [PubMed]
- Sgroi MD, Narayan RR, Lane JS, et al. Vascular reconstruction plays an important role in the treatment of pancreatic adenocarcinoma. J Vasc Surg 2015;61:475-80. [Crossref] [PubMed]
- Barreto SG, Windsor JA. Justifying vein resection with pancreatoduodenectomy. Lancet Oncol 2016;17:e118-24. [Crossref] [PubMed]
- Tseng JF. Proceed with caution: vascular resection at pancreaticoduodenectomy. Ann Surg Oncol 2012;19:4001-2. [Crossref] [PubMed]
- Castleberry AW, White RR, De La Fuente SG, et al. The impact of vascular resection on early postoperative outcomes after pancreaticoduodenectomy: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Ann Surg Oncol 2012;19:4068-77. [Crossref] [PubMed]
- Gurusamy KS, Kumar S, Davidson BR, et al. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev 2014.CD010244. [PubMed]
- Christians KK, Pilgrim CHC, Tsai S, et al. Arterial resection at the time of pancreatectomy for cancer. Surgery 2014;155:919-26. [Crossref] [PubMed]
- Fujii T, Nakao A, Yamada S, et al. Vein resections >3 cm during pancreatectomy are associated with poor 1-year patency rates. Surgery 2015;157:708-15. [Crossref] [PubMed]
- Krepline AN, Christians KK, Duelge K, et al. Patency rates of portal vein/superior mesenteric vein reconstruction after pancreatectomy for pancreatic cancer. J Gastrointest Surg 2014;18:2016-25. [Crossref] [PubMed]
- Javed AA, Bleich K, Bagante F, et al. Pancreaticoduodenectomy with venous resection and reconstruction: current surgical techniques and associated postoperative imaging findings. Abdom Radiol (NY) 2017. [Epub ahead of print]. [Crossref] [PubMed]