Is a simplified TNM staging system more clinically relevant than the American Joint Committee on Cancer system for the follicular variant of papillary thyroid cancer?
Introduction
The follicular variant of papillary thyroid carcinoma (FVPTC) is histologically characterized by follicular cell growth patterns and the presence of nuclear features of classical PTC (CPTC) (1,2). FVPTC is the most common subtype of malignant papillary thyroid tumor apart from CPTC, accounting for up to 23% of all PTCs (2,3). Recent statistics have suggested that the incidence of FVPTC is increasing steadily, especially in Western countries (4-6), which has drawn increased attention for the diagnosis, management, and prognosis of FVPTC.
The current consensus among thyroid academics is that there are only a few differences between FVPTC and CPTC; moreover, the overall management of the two malignancies are similar, and patients with FVPTC and CPTC have identical long-term outcomes (7-12). However, research have shown that despite these similarities, patients with FVPTC present clinically with more favorable clinicopathologic features and are stratified into lower and less-aggressive tumor risk categories (13). For example, a recent largescale multinational study suggested that FVPTC was associated with lower rates of extrathyroidal invasion, lymph node metastasis, disease recurrence, and mortality when compared with CPTC (14). However, there was no difference between CPTC and FVPTC in the use of clinical radioiodine-131 treatment (14), despite the potentially poorer prognosis typically associated with this therapy. Therefore, patients with FVPTC might expect better prognosis with tailored disease management.
According to the American Thyroid Association Management guidelines, the goals of initial therapy for patients with PTC include accurate disease staging and risk stratification, the minimization of adverse and unnecessary therapy, and the achievement of a favorable prognosis (15). Despite these recommended goals, the most recent 8th edition of the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) staging system, which is the most widely used risk stratification system, does not distinguish FVPTC from PTC (16). Thus, the present study aimed to develop a more accurate and clinically relevant TNM staging system for patients with FVPTC.
Methods
Patients and database
For this study, we obtained the data of patients with FVPTC who were included in the openly accessible Surveillance, Epidemiology, and End Results (SEER) database (National Cancer Institute, Bethesda, MD, USA) between 2010 and 2015. Since SEER is a publicly available database with anonymized data, no ethical review was required. Additionally, a data use agreement was signed for this project.
Accounting for the favorable prognosis of FVPTC, we selected overall survival (OS) data, rather than cancer-specific survival (CSS). Furthermore, we excluded 644 cases in the following manner: 632 cases with recorded categories of T0, TX, NX, N1NOS, or T4NOS (In SEER database, a status described as “N1NOS” or “T4NOS” is distinguished, but it does not exist in the TNM criteria defined in the TNM/AJCC staging system. Thus, we excluded cases that recorded categories of N1NOS or T4NOS), 11 cases with unclear survival duration, and 1 case in which the patient died during the 83rd month of follow-up of an unknown cause. The following data were collected for all patients: age at diagnosis, year of diagnosis, sex, race, T/N/M category, TNM stage according to the 8th edition of AJCC, tumor size, number of tumor foci, extension, radiation status, and surgical modality. Missing or unclear data were treated as user missing values.
Development process
We initially divided all cases into 2 groups using the cut-off age of 55 years. Next, we divided the total patient sample into 47 groups according to the T, N, and M categories. In this step, we excluded groups which contained cases below 10 and with no mortality as follows: age <55: T1N1aM1 (n=1), T1N1bM1 (n=2), T2N0M1 (n=1), T2N1aM1 (n=1), T2N1bM1 (n=1), T3N1aM1 (n=4), T4N0M1 (n=3), T4N1aM1 (n=1); age ≥55: T4N1bM1 (n=9), T1N1aM1 (n=6), T1N1bM1 (n=2), T2N1bM1 (n=4). After filtering out 679 cases from the total data, 17,628 patients were included in the study.
Then, we divided these groups into three new proposed stages based on the results of the clinical experiences and Kaplan-Meier (K-M) survival curves. Furthermore, we calculated the probability of mortality per 1,000-person-years. Cox proportional hazards models were used to assess the variables associated with prognosis in the three final stages after adjusting for age at diagnosis, year of diagnosis, sex, race, tumor size, number of tumor foci, tumor extension, radiation, and surgical modality.
Statistical analysis
The demographic and clinical information are summarized as frequencies, proportions, and mean values ± standard deviations, as appropriate. As noted above, K-M curves, Cox proportional hazards models, and mortality per 1,000-person-year were used in the survival analyses. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA), Stata/SE version 15 (Stata Corp, College Station, TX, USA), GraphPad Prism version 7 (GraphPad Software Inc., La Jolla, CA, USA), or MATLAB version 2018a (MathWorks, Cambridge University Press, Cambridge, UK).
Results
Patient demographics
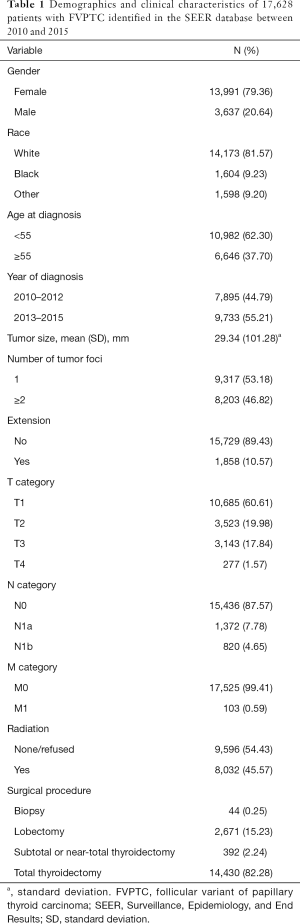
The demographic and clinical characteristics of the included patients are summarized in Table 1. The 17,628 cases included 13,991 female and 3,637 male patients (approximate female:male ratio, 3.85:1). At diagnosis, 10,982 patients were younger than 55 years, and 6,646 were 55 years or old (approximate ratio, 1.65:1). Moreover, 10,685, 3,523, 3,143, and 277 patients had T1, T2, T3, or T4 diseases, respectively; 15,436, 1,372, and 820 patients had N0, N1a, or N1b disease, respectively; and 17,525 and 103 patients had M0 or M1 disease, respectively.
Full table
The proposed TNM staging system
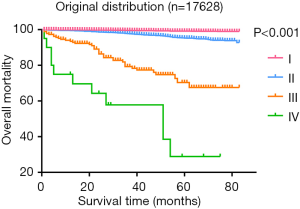
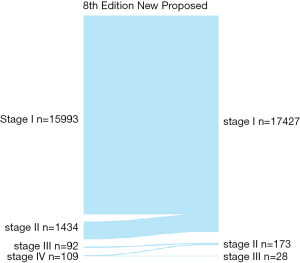
Patients were divided into 47 groups, as described in the materials and methods section (see Table S1). Figure 1 presents the survival status of all the patients based on the distribution into these 47 groups. After excluding groups that contained cases below 10 and no mortality, we then used the survival trends to classify the remaining groups into four stages (Figure 2), which we termed “original distribution”. However, a few groups with fewer than 10 cases and with mortality were included because of the clinical and statistical significance.
Full table
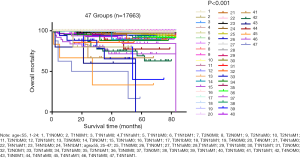
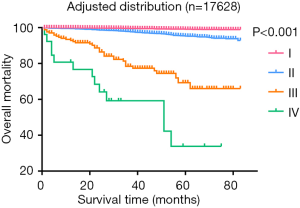
Additionally, the statistical results of the group of patients <55 years with T3/N0/M1 and containing ten cases, were inconsistent with the clinical prognosis because patients in this group had a more favorable prognosis than those in the stage II groups in clinical settings, which might be attributable to an insufficient number of cases. We then used the clinical experiences to adjust this distribution, treating those aged <55 years with T3/N0/M1 disease as stage I, which we termed “adjusted distribution”, as shown in Figure 3. We considered patient aged ≥55 years and with a distant metastasis as high-risk factor. We assigned patients who met those criteria to stage IV. As shown in Figure 4, however, this division decreased the difference between stages III and IV when compared with the adjusted distribution.
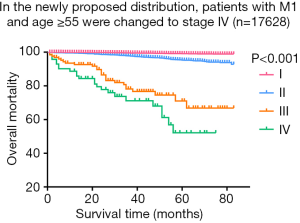
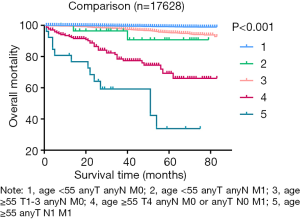
As shown in Figure 3, we observed few differences between stage I, which was composed of groups of age <55 and any T/any N/M0, and stage II, which consisted of groups from age <55 and any T/any N/M1 or age ≥55 and T1-3/any N/M0. Then, we analyzed the shape of Figure 5, which revealed that the trends of curves of those three groups were similar. This indicated to us that those three groups share similar mortality rates, and the adjusted Cox analysis and mortality per 1,000-person-year of these three groups are shown in Table S2 and S3, respectively. Overall mortality per 1,000-person-year of all 47 groups is also available in Table S4. Therefore, our newly proposed staging system combines stages I and II in the adjusted distribution as new stage I. In our new system, stage I is defined as an age <55 years and any T/N/M or an age ≥55 years and T1-3/any N/M0. Stage II is defined as an age ≥55 years and T4/any N/M0 or any T/N0/M1. Stage III is defined as aged ≥55 years and any T/N1/M1. A comparison of the group distribution of original distribution, adjusted distribution, and the newly proposed staging system is shown in Table 2.
Full table

Full table
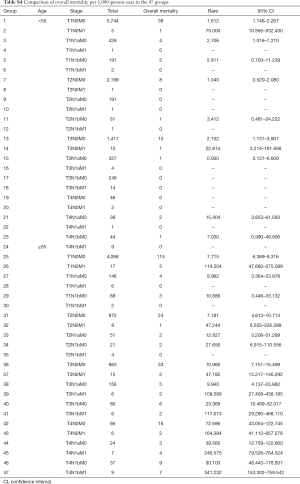
Full table
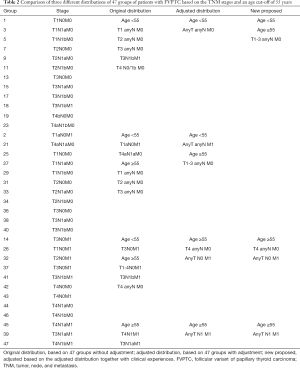
Full table
Predictive ability of the new proposed TNM staging system
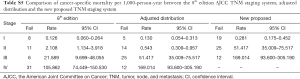
After formatting our newly proposed TNM staging system, we verified its accuracy by comparing the K-M curves of the estimated OS, and CSS generated from the data stratified by the 8th edition of AJCC and our newly proposed staging system (Figures 6 and 7), respectively. The downward trends of all the curves based on the new proposed staging system were more even and distinctive. The distributions and frequencies of cases are shown in Table 3 and Figure 8. Stage I, II, and III in the newly proposed system included 17,427, 173, and 28 patients, respectively. Accordingly, the new proposed system provided a superior representation of the gradient of disease classification.
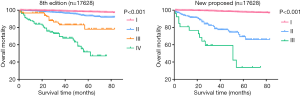
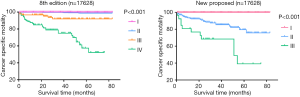

Full table
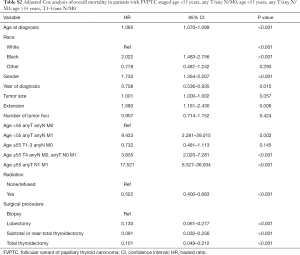
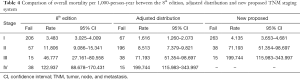
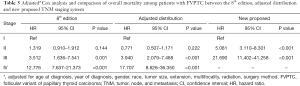
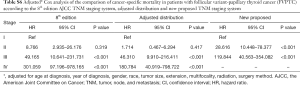
Tables 4 and 5 present a comparison of the overall mortality rate per 1,000-person-year and the results of Cox analysis based on the 8th edition AJCC staging system, adjusted distribution, and newly proposed system. The overall mortality rates per 1,000-person-year for new stage I, II, and III were 4.135 [95% confidence interval (CI): 3.653–4.681], 71.193 (95% CI: 51.354–98.697), and 199.744 (95% CI: 115.983–343.997), respectively. The adjusted hazard ratios (HRs) for the new II, and III (reference: stage I) were 5.081 (95% CI: 3.110–8.301; P<0.001) and 21.690 (95% CI: 11.402–41.258, P<0.001), respectively. Furthermore, comparison of cancer-specific mortality per 1,000-person-year based on 8th edition AJCC staging system, adjusted distribution, and newly proposed system were shown in Table S5. Table S6 presents the results based on CSS data.
Full table
Full table
Full table
Full table
Discussion
The AJCC staging system is used for the risk stratification of various carcinomas. This system is primarily based up on the anatomic extent of cancer and is continuously updated to remain relevant to current clinical practice and advances in cancer prognosis (17). However, this system is suboptimal for the risk stratification of FVPTC, despite the status of this malignancy as the second common PTC subtype. Accordingly, the AJCC system has failed to adapt to the concept of precision medicine advocated by the ATA, wherein the need for adequate treatment is balanced against the risk of overtreatment (15). Accordingly, we proposed a new staging system for patients with FVPTC.
In this study, we used a sample of patients included in the SEER database, which has been recognized annually by the North American Association of Central Cancer Registries for its completeness and accuracy (18), as well as various statistical methods and clinical factors. After various calculations, comparisons and modeling, we hereby propose a new three-stage system for FVPTC, as described in the Results. This new proposed simplified staging system provides better stratification of low-, medium-, and high-risk patients than that of the AJCC staging system. The conversion of a four-stage system to a three-stage system is the most significant change proposed in this work. Our proposed system is consistent with an earlier observation by Jukkola et al. indicating that the AJCC TNM staging system adequately distinguishes stages I and IV, but less clearly distinguishes the intermediate-risk groups (stages II and III) (19). Jukkola et al. found that the relevance of the TNM classification improved after combining stages I and II (19). Similarly, we pooled stage I and II into our new stage I.
We additionally classified all patients younger than 55 years into stage I, regardless of their N or M category. This was consistent with an earlier observation by Zaydfudim et al. that patients with PTCs younger than 45-year-old did not affect survival rates (20). Kim et al. compared three subtypes of FVPTC and suggested that the clinicopathologic behavior of noninvasive encapsulated FVPTC was similar to that of invasive encapsulated FVPTC but distinct from that of infiltrative FVPTC. Their observation indicated that the combination of lymph node and distant metastases might indicate a worse prognosis than those of either alone (21), consistent with our proposed stage III. Furthermore, patients categorized as our new stage III, aged ≥55 years with lymph node and distant metastases, also corresponded to the high-risk category and were recommended to undergo radioactive iodine therapy remnant ablation. In contrast, patients classified as stage I may undergo simple lobectomy (15,22). In summary, our newly proposed TNM staging system is more clinically practical than the existing system.
The MACIS staging system considers metastasis, age, completeness of resection, invasion, and size when predicting the mortality of patients with PTC after primary surgery (23). The QTNM staging system aims to provide a simple risk stratification method but may not contain a sufficient number of effective factors (24,25). In contrast, our newly proposed staging system is based on the existing AJCC TNM staging system and the overall mortality associated with FVPTC. The advantages of this system include its simplicity and clinical practical, as well as the ability to provide a risk classification at the initial diagnosis. Consequently, this system could be highly valuable when estimating the subsequent management and prognosis.
Despite the aforementioned advantages, our study had some limitations. Genetic, environmental, and biological factors should be considered in staging models. However, the importance of these factors remains controversial. Accordingly, we aim to follow the mainstream consensus regarding thyroid carcinoma and will add additional relevant factors to our staging system in a stepwise manner over time to continue the facilitation of risk stratification, management, and prognosis for FVPTC. We also note that our newly proposed staging system is based on the SEER database, which includes a primarily North American population. This may affect the generalizability of our system.
In conclusion, the present study aimed to develop a new staging system that could be used for risk stratification of FVPTC. Compared to the 8th edition of the AJCC staging system, our newly proposed system ca provided more accurate risk stratification for patients with FVPTC, as demonstrated by actual survival and mortality outcomes. This new model may thus help guide more personalized treatment for these patients. However, this preliminary study leaves some questions to be answered, and extensive trials with more diverse patient populations are needed to verify our conclusions.
Acknowledgments
Funding: The authors are sponsored by the Shanghai Pujiang Program (2019PJD040) and the Pandeng Program of Shanghai Tenth People's Hospital (2018SYPDRC018).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.111). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Brahim N, Asa SL. Papillary thyroid carcinoma: an overview. Arch Pathol Lab Med 2006;130:1057-62. [PubMed]
- Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol 2011;5:51-6. [Crossref] [PubMed]
- Tunca F, Sormaz IC, Iscan Y, et al. Comparison of histopathological features and prognosis of classical and follicular variant papillary thyroid carcinoma. J Endocrinol Invest 2015;38:1327-34. [Crossref] [PubMed]
- Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014;99:E276-85. [Crossref] [PubMed]
- Albores-Saavedra J, Henson DE, Glazer E, et al. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype--papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol 2007;18:1-7. [Crossref] [PubMed]
- Hong AR, Lim JA, Kim TH, et al. The Frequency and Clinical Implications of the BRAF(V600E) Mutation in Papillary Thyroid Cancer Patients in Korea Over the Past Two Decades. Endocrinol Metab (Seoul) 2014;29:505-13. [Crossref] [PubMed]
- Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006;154:787-803. [Crossref] [PubMed]
- Burningham AR, Krishnan J, Davidson BJ, et al. Papillary and follicular variant of papillary carcinoma of the thyroid: Initial presentation and response to therapy. Otolaryngol Head Neck Surg 2005;132:840-4. [Crossref] [PubMed]
- Chang HY, Lin JD, Chou SC, et al. Clinical presentations and outcomes of surgical treatment of follicular variant of the papillary thyroid carcinomas. Jpn J Clin Oncol 2006;36:688-93. [Crossref] [PubMed]
- Hagag P, Hod N, Kummer E, et al. Follicular variant of papillary thyroid carcinoma: clinical-pathological characterization and long-term follow-up. Cancer J 2006;12:275-82. [Crossref] [PubMed]
- Yu XM, Schneider DF, Leverson G, et al. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: a population-based study of 10,740 cases. Thyroid 2013;23:1263-8. [Crossref] [PubMed]
- LiVolsi VA. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer 2003;98:1997; author reply 1998.
- Lang BH, Lo CY, Chan WF, et al. Classical and follicular variant of papillary thyroid carcinoma: a comparative study on clinicopathologic features and long-term outcome. World J Surg 2006;30:752-8. [Crossref] [PubMed]
- Shi X, Liu R, Basolo F, et al. Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. J Clin Endocrinol Metab 2016;101:264-74. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Edge SB, Byrd DR, Brookland RK, et al. AJCC Cancer Staging Manual 8th Edition. Springer, 2017.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Gan T, Huang B, Chen Q, et al. Risk of Recurrence in Differentiated Thyroid Cancer: A Population-Based Comparison of the 7th and 8th Editions of the American Joint Committee on Cancer Staging Systems. Ann Surg Oncol 2019;26:2703-10.
- Jukkola A, Bloigu R, Ebeling T, et al. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer 2004;11:571-9. [Crossref] [PubMed]
- Zaydfudim V, Feurer ID, Griffin MR, et al. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008;144:1070-7; discussion 1077-8. [Crossref] [PubMed]
- Kim MJ, Won JK, Jung KC, et al. Clinical Characteristics of Subtypes of Follicular Variant Papillary Thyroid Carcinoma. Thyroid 2018;28:311-8. [Crossref] [PubMed]
- Luster M, Aktolun C, Amendoeira I, et al. European Perspective on 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: Proceedings of an Interactive International Symposium. Thyroid 2019;29:7-26. [Crossref] [PubMed]
- Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993;114:1050-7; discussion 1057-8. [PubMed]
- Onitilo AA, Engel JM, Lundgren CI, et al. Simplifying the TNM system for clinical use in differentiated thyroid cancer. J Clin Oncol 2009;27:1872-8. [Crossref] [PubMed]
- Mankarios D, Baade P, Youl P, et al. Validation of the QTNM staging system for cancer-specific survival in patients with differentiated thyroid cancer. Endocrine 2014;46:300-8. [Crossref] [PubMed]