The diagnostic value of the combination of Golgi protein 73, glypican-3 and alpha-fetoprotein in hepatocellular carcinoma: a diagnostic meta-analysis
Introduction
Globally, liver cancer ranks as the seventh most prevalent primary malignant tumor and is the third-biggest contributor to cancer-related mortality (1), with hepatocellular carcinoma (HCC) accounting for more than 90% of liver cancer cases (2). On account of HCC’s highly aggressive and insidious nature and a lack of effective and early diagnostic methods, a large proportion of HCC cases are identified when the patients are already at an advanced stage, at which point curative treatments are not viable, resulting in a fairly poor prognosis (3). Consequently, early detection and effective treatment are extremely important to improve the overall survival of life of patients with HCC.
Since the 1970s, alpha-fetoprotein (AFP) has remained the most commonly used serum biomarker in the screening and diagnosis of HCC in clinical practice. However, previous studies have found that a number of patients with benign hepatic diseases may have an elevated level of AFP, while no elevated level of AFP has been detected in patients with HCC. Because of its poor sensitivity and specificity, especially during early-stage HCC, the diagnostic performance of AFP is not satisfactory, and there is an urgent need for novel biomarkers which could complement or even replace AFP (4-6).
A type of glycosyl-phosphatidyl-inositol anchored heparan sulfate proteoglycan, which are subordinate to the glypican family, glypican-3 (GPC-3) has been identified as being closely related to the proliferation and metastasis of cancerous cells (7,8). Normal human tissues see low expression of GPC-3, but in diseased liver tissues, especially HCC tissue, previous studies have found the protein to be overexpressed (9). Additionally, there has been no correlation discovered between the levels of GPC3 and AFP, which illustrates that both elements are functionally independent (10). In a normal liver, the expression of Golgi protein 73 (GP73), a resident Golgi-specific membrane protein, principally occurs in the epithelial cells of the bile duct, but with chronic liver diseases, in particular HCC, there is a marked increase in its expression (11). The value of serum GP73 as a diagnostic indicator has been shown to be higher than that of AFP (12).
However, the results of studies relating to the performance of the combined application of serum GP73, GPC3 and AFP in the diagnosis of HCC remain controversial. This meta-analysis aimed to summarize and analyze results from studies focused on the diagnostic performance of the combined application of serum GP73, GPC3 and AFP for HCC diagnosis.
Methods
Search strategy and study selection
To identify relevant studies, a comprehensive literature review was performed using the, Web of Science, Cochrane Library, Embase, Chinese Biomedical Literature Database and China National Knowledge Infrastructure databases. Studies conducted up to 1 November, 2019 were filtered using the following search terms: (I) GP73: GP73, Golgi protein 73, Golgi phosphoprotein 2, Golgi membrane protein 1; (II) GPC3: glypican-3, glypican3, glypican 3; (III) AFP and alpha Fetoprotein (IV) HCC: liver cancer, HCC, liver neoplasm, hepatic neoplasm. Consideration was also given to the reference lists of the relevant studies and publications, until no possible articles could be found. The articles were independently reviewed by two reviewers (Shoujie Zhao and Desha Zheng), and any disagreements were resolved by discussion.
The included criteria were as follows: (I) all patients included were diagnosed with HCC by contrast-enhanced magnetic resonance imaging (MRI) and computed tomography (CT) according to the guidelines of the American Association for the Study of the Liver Disease and European Association for the Study of Liver Disease (AASLD-EASL); (II) comparison of the diagnostic accuracy of the combination of GP73, GPC-3 and AFP either with a single method or alliance in pairs; (III) the sensitivity and specificity of each combination should have been presented directly or converted by calculating the original data in the studies.
If studies met any of the following criteria, they were not considered appropriate for inclusion in our meta-analysis: (I) repetitive studies, narrative reviews, letters, comments, case reports or studies unrelated to our topic; (II) no control groups; (III) experiments on laboratory animals and cultured cells; (IV) studies consisting of an evaluation of serum maker levels by messenger RNA, DNA or DNA polymorphism analysis; (V) a lack of extractable data.
Data extraction
Two independent investigators extracted the following data from the articles that qualified for inclusion in our meta-analysis: the first author’s name, the year of publication, the number of HCC patients and controls, the type of marker assay, and original data relating to sensitivity and specificity [the amount of true positive (TP), false negative (FP), true negative (TN), and false positive (FN) results]. Furthermore, the following formulas were used to calculate the number of TP, FP, FN, and TN results: TP = number of HCC patients × sensitivity; FP = number of non-HCC patients × (1 − specificity); FN = number of HCC patients × (1 − sensitivity); TN = number of non-HCC patients × specificity; all disagreements relating to data extraction were discussed with a third independent researcher to reach a consensus.
Assessment of methodological quality
The assessment of the quality of the included studies was determined according to the Quality Assessment of Studies of Diagnostic Accuracy included in Systematic Reviews (QUADAS) checklist recommended by the Cochrane Collaboration (13). To assist with our risk-of-bias assessment, 14 items were applied and classified as ‘yes’ if reported, ‘no’ if not reported, or ‘unclear’ if information was not sufficient enough to inform a precise judgment. Each of the 14 items that assessed risk of bias was scored as ‘high’, ‘low’, or ‘unclear’.
Statistical analysis
Statistical analyses were conducted by Stata 12.0 and Meta-Disc 1.4 software. Pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR), as well as their 95% confidence intervals (95% CIs), were calculated and displayed in the form of forest plots. Diagnostic accuracy was determined using the summary receiver operating characteristic curve (SROC) and its AUC. The evaluation of the extracted data’s heterogeneity was conducted according to I2 value. Insignificant heterogeneity was defined as I2 value <50% and P value >0.1. In the absence of heterogeneity, meta-analysis was carried out using a fixed effects model, whereas when heterogeneity was identified, a random effects model was used. Spearman’s rank correlation was used to measure whether the threshold effect resulted in heterogeneity. If Spearman’s correlation coefficient was around 1 and P≤0.05, we deemed the threshold effect to exist.
Meta-regression was conducted to analyze heterogeneity if there was no threshold effect. Publication bias was examined using Deeks’ funnel plot, with a P value <0.05 suggesting an underlying publication bias. The assessment of the clinical practicability of the joint detection of GP73, GPC-3 and AFP was carried out using the Fagan nomogram and likelihood matrix.
Results
Study selection and study-quality analysis
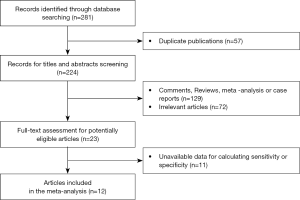
After carrying out a literature search of the databases mentioned previously, 281 potentially relevant articles were initially identified. Among these articles, 57 were excluded due to duplication. After the examination of titles and abstracts, a further 201 studies were excluded. The remaining 23 studies were selected for full-text screening. Out of these, 12 studies were eventually deemed eligible for inclusion in this meta-analysis (14-25). The study recruitment flowchart is shown in Figure 1. The clinical features of the 12 included articles, as well as their methodology, are set out in Table 1. The results of the 12 included studies’ quality assessments, conducted according to QUADAS, are shown in Table 2.
Full table

Full table
Summary diagnostic value for HCC
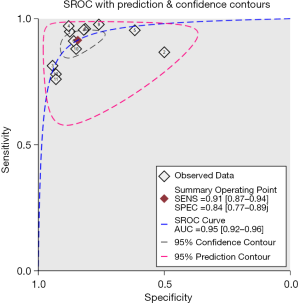
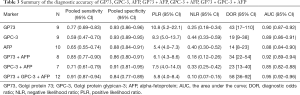
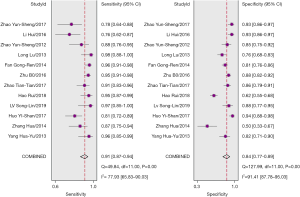
The pooled sensitivity and specificity for the combination of GP73, GPC3, and AFP in discriminating HCC from non-HCC were 0.91 (95% CI: 0.87–0.94, P<0.001) and 0.84 (95% CI: 0.77–0.89, P<0.001), respectively (Figure 2). The pooled PLR and NLR were 5.83 (95% CI: 4.05–8.40, P<0.001) and 0.10 (95% CI: 0.07–0.15, P<0.001), respectively. The DOR was 57.51 (95% CI: 35.92–92.08, P<0.001). SROC curves were used to plot the articles’ sensitivity and specificity (Figure 3), and the AUC value was 0.95 (95% CI: 0.92–0.96, P<0.001). The DOR of the joint detection of GP73, GPC-3 and AFP was highest, while it was the lowest for AFP alone (Table 3).
Full table
Evaluation of clinical utility
The clinical utility of the combination of GP73, GPC3 and AFP was assessed by utilizing likelihood ratios to establish a Fagan nomogram. The Fagan nomogram demonstrated an increase of 35.3% in the post-test probability but a decrease of 40.9% in patients based on 50% pre-test probability (Figure 4A). The combination of GP73, GPC3 and AFP proved to be particularly accurate, with a 65.9% probability of correctly distinguishing between benign and malignant liver lesions after a positive report when the pre-test probability was 25% and a reduction in the probability of disease to as low as 3.2% when a negative test result occurred (Figure 4B). Additionally, diagnosis of patients who had negative results, had a 23.1% post-test probability of being wrong whereas the pre-test probability stood at 75%; however, for patients with a positive test, the probability of correctly diagnosing malignant liver lesions exceeded 90% (Figure 4C).

Test for heterogeneity
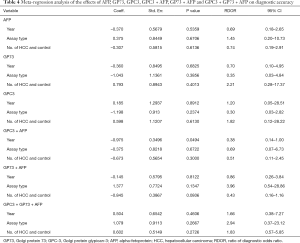
Significant heterogeneity existed among the articles enrolled in this meta-analysis. Spearman’s correlation coefficient, which examined the threshold effect, was 0.501, P=0.097) demonstrated that heterogeneity did not come as a result of the cut-off point. Furthermore, the year of publication, sample size and type of assay were suggested by meta-regression analysis to have not influenced the result of heterogeneity (Table 4).
Full table
Publication bias and sensitivity analyses
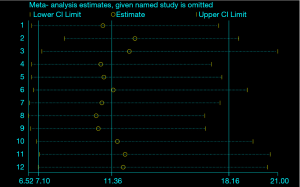
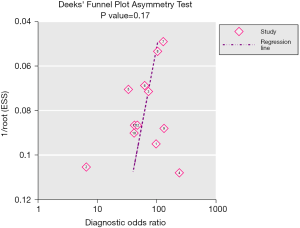
There was no evidence, based on the result of Deeks’ funnel plot, to suggest any significant publication bias for the joint identification of GP73, GPC3 and AFP (P=0.17, Figure 5). For sensitivity analyses, one study per time was omitted to check if individual studies had affected the final result. The results were not materially altered, which indicated that no study had exclusively contributed to the publication bias and that the pooled results were steady (Figure 6).
Discussion
Through this meta-analysis, we aimed to carry out a systematic evaluation of the diagnostic accuracy of the combination of GP73, GPC-3 and AFP in discriminating HCC patients from non-HCC patients. The findings of this meta-analysis demonstrated that joint detection had a high diagnostic performance in diagnosing HCC and ruling out non-HCC patients, when compared with the performances of GP73, GPC-3, or AFP alone, or in pairs.
Due to the aggressive nature and poor prognosis of HCC, early detection is crucial in improving the survival of patients of the disease. For effective and accurate diagnosis of HCC, histopathological assessment remains the benchmark, despite its invasive nature and although, in comparison, serum markers show high superiority to some extent. Traditionally, ultrasonography and serum AFP remain commonly used methods of detecting early-stage HCC in clinical practice. However, on account of its low sensitivity and specificity, the clinic effectiveness of AFP in the diagnosis of HCC is unsatisfactory. GP73 and GPC-3 have also been put forward as serum markers for HCC in many studies which showed their high discriminatory ability.
In this study, we examined the diagnostic value of the joint detection of GP73, GPC-3 and AFP. To avoid a large number of potentially confusing factors in our comparisons, we limited the included studies to those which measured GP73, GPC3 and AFP in patients with similar characteristics. Tests with high sensitivity and low NLR indicate that patients suspected of having the disease could be screened according to series test. A low NLR value illustrates the capability of the diagnostic methods in excluding non-HCC diseases. According to the pooled sensitivity and NLR, a combination of these three biomarkers showed better diagnostic ability than that of GP73, GPC-3, and AFP alone or in pairs. DOR was used to assess the accuracy, because it is a single measure of diagnostic value which takes into account sensitivity and specificity and LR positive and LR negative. DOR is determined to be the ratio of the odds of positive test results of participants who have a disease to the odds of positive test results of participants who do not have the same disease (26). In this meta-analysis, a combination of these three biomarkers showed the highest DOR, suggesting it was more helpful for the early diagnosis of HCC than either GP73, GPC-3, and AFP alone or in pairs.
The SROC curve along with AUC are vital indexes in the assessment of diagnostic accuracy as part of diagnostic meta-analyses. The AUC ranges from 0 to 1. When the AUC is 0, it illustrates a test lacks accuracy as a method of diagnosis. When the AUC is 1, however, a test that accurately discriminates between all cases and non-cases is confirmed. In the SROC curve analysis of this meta-analysis, the AUC value of a combination of these three biomarkers was 0.95, which indicated that joint detection showed a higher diagnostic accuracy than GP73, GPC-3, and AFP alone or in pairs. Furthermore, the Fagan nomograms also showed that a combination of these three biomarkers could be helpful for early HCC diagnosis.
In this meta-analysis, large heterogeneity was observed and the reasons for heterogeneity were investigated using meta-regression analysis. No remarkable change was observed when any article was removed from the study, which indicated not one individual study had an effect on the heterogeneity. Additionally, no threshold effect was detected from the SROC curve. In addition, the meta-regression method was performed to explore the heterogeneity according to the studies’ characteristics, but no statistical difference was discovered. This meta-analysis also failed to reveal all of the possible causes of heterogeneity seen among the enrolled studies, due to the enrolled studies lacking important elements in their design.
Several limitations in our meta-analysis should be taken into account. First, significant heterogeneity was observed among the included studies. However, there was no statistically significant effect caused by assay type, number of patients or publication year in terms of diagnostic accuracy. Due to the lack of available information on design and patient population, larger sample sizes and multicenter RCTs are required before our results can be confirmed and the heterogeneity further explored. Furthermore, most of the study population in this meta-analysis were Chinese patients with hepatitis B viral infections as the etiology of HCC, which is different from patients in most Western countries, where the etiologies of HCC are mainly hepatitis C virus infection and alcoholic liver disease. Therefore, a cautious approach should be taken towards generalizing our findings and future prospective studies are needed.
Conclusions
Our meta-analysis demonstrated that the diagnostic value of the joint detection of GP73, GPC-3 and AFP was significantly higher than that of GP73, GPC-3, and AFP alone or in pairs. The results of this meta-analysis should be investigated by further studies in order to select high-risk groups and to improve the capacity of early diagnosis of early-stage HCC patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.89). LL serves as the unpaid editorial board member of Annals of Translational Medicine from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Raoul JL, Kudo M, Finn RS, et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat Rev 2018;68:16-24. [Crossref] [PubMed]
- Jing JS, Ye W, Jiang YK, et al. The Value of GPC3 and GP73 in Clinical Diagnosis of Hepatocellular Carcinoma. Clin Lab 2017;63:1903-09. [Crossref] [PubMed]
- Edoo MIA, Chutturghoon VK, Wusu-Ansah GK, et al. Serum Biomarkers AFP, CEA and CA19-9 Combined Detection for Early Diagnosis of Hepatocellular Carcinoma. Iran J Public Health 2019;48:314-22. [PubMed]
- Wang Y, Zhang C, Zhang P, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med 2018;7:1670-9. [Crossref] [PubMed]
- El-Saadany S, El-Demerdash T, Helmy A, et al. Diagnostic Value of Glypican-3 for Hepatocellular Carcinoma. Asian Pac J Cancer Prev 2018;19:811-7. [PubMed]
- Wang L, Yao M, Pan LH, et al. Glypican-3 is a biomarker and a therapeutic target of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2015;14:361-6. [Crossref] [PubMed]
- Chen IP, Ariizumi S, Nakano M, et al. Positive glypican-3 expression in early hepatocellular carcinoma predicts recurrence after hepatectomy. J Gastroenterol 2014;49:117-25. [Crossref] [PubMed]
- Tangkijvanich P, Chanmee T, Komtong S, et al. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol 2010;25:129-37. [Crossref] [PubMed]
- Ke MY, Wu XN, Zhang Y, et al. Serum GP73 predicts posthepatectomy outcomes in patients with hepatocellular carcinoma. J Transl Med 2019;17:140. [Crossref] [PubMed]
- Zhou Y, Yin X, Ying J, et al. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer 2012;12:17. [Crossref] [PubMed]
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [Crossref] [PubMed]
- Fan GR, Wang WF, Han JQ, et al. Application of the combination detection of serum AFP, GP73 and GPC3 on the diagnosis of primary hepatic carcinoma. Laboratory Medicine 2014;29:884-8.
- Hao R, Zheng XS, Zhang LL, et al. Application value of five serum markers in the diagnosis of hepatocellular carcinoma. Chin J Ctrl Endem Dis. 2018;33:125-7.
- Huo YS, Li DW, Feng YC, et al. Application value of GPC3 and GP73 in the diagnosis of hepatocellular carcinoma. Int J Lab Med 2017;38:2462-4.
- Li H, Huang XL, Liu Y. Significance of combined tests of serum golgi glycoprotein 73 and other biomarkers in diagnosis of small primary hepatocellular carcinoma. Labeled Immunoassays and Clin Med 2016;23:395-9.
- Long L, Chen Z, Wang K, et al. Value of GPC3, GP73, AFP-L3 and AFP detection in the diagnosis of primary hepatic carcinoma. Chin J of Mod Med 2013;23:46-50.
- Lv S. Diagnosis accuracy of GPC3, GP73 and AFP detection in the diagnosis of primary hepatocellular carcinoma. Mod Diagn Treat 2019;30:1128-9.
- Yang HY, Wang YF, Xu HF, et al. Utility of alpha-fetoprotein, golgi orotein 73 and glypican-3 alone or in combination as biomarkers of hepatocellular carcinoma. Oncol Prog 2013;11:249-53.
- Zhang H, Li YX, Le Y, et al. The clinical value of the combined detection of AFP, GPC-3 and GP73 in patients with HCC. Labeled Immunoassays and Clin Med 2014;21:132-5.
- Zhao TT, Li HC, Peng H, et al. Value of combined detection of AFU, AFP, GP73 and GPC3 in the diagnosis of primary hepatic carcinoma. Progress in Mod Bio 2017;17:1941-4.
- Zhao YS, Wang M, Li JL, et al. Clincial significance of serum GP73, DCP, GPC-3 and AFP tests in diagnosis of primary hepatic carcinoma. Chin Gengeral Practice 2012;15:3730-3.
- Zhao YS, Wang CH, Gao Q, et al. Value and verification of combined tests of serum GP73, AFP-L3, GPC-3 and DCP in diagnosis of low concentration AFP of small primary hepatocellular carcinoma. Lab Med Clin 2017;14:19-23.
- Zhu B, Xiao YX, Peng YS, et al. Utility of GP73, GPC3 and AFP in diagnosis of primary hepatocellular carcinma. Chongqing Med 2016;45:1367-9.
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]