Efficacy evaluation of the combination therapy of sorafenib and transarterial chemoembolization for unresectable HCC: a systematic review and meta-analysis of comparative studies
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths and the fifth most common malignant tumor worldwide (1). Asian countries account for three-quarters of HCC-related deaths and, in most countries, 70 percent of patients are infected with chronic hepatitis B virus (HBV) (2,3).
The Barcelona Clinic Liver Cancer (BCLC) staging system is the most extensively adopted HCC classification system worldwide. According to the BCLC staging system, the major therapies for BCLC-A HCC patients are surgical section, liver transplantation and radiofrequency ablation. For BCLC-B patients, transarterial chemoembolization (TACE) is the recommended standard therapy, whereas for BCLC-C patients, sorafenib is the recommended targeted drug (4). Most patients are at BCLC-B/C stage when they are diagnosed.
Most previous clinical trials have proved that TACE can improve the survival of BCLC-B patients (5-8). It allows the direct delivery of the anticancer therapy to the tumor feeding arteries by preferentially blocking the arterial blood supply of liver tumors (9). However, due the potentially damaging effects of TACE on the hepatic arterial system, the long-term benefit is less effective for patients with worsening liver function (10). Moreover, after TACE treatment, overexpression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) may lead to high recurrence of HCC (11). As a multi-kinase inhibitor, sorafenib targets and inhibits multiple the signal transduction pathways of HCC development and restrains tumor cell proliferation and angiogenesis (12). In addition, it works to inhibit VEGF and PDGF receptors (13). Therefore, in theory, by combining sorafenib with TACE, the expression of VEGF and PDGF after TACE may be significantly decreased (14). It remains a reasonable hypothesis whether sorafenib could regulate the upregulation of TACE-induced angiogenic factors and potentially enhance its efficacy (15).
The efficacy of combination therapy has already been investigated by previous systematic reviews. However, most of the included studies were non-comparative studies (16-18), and only a small proportion were randomized controlled trials (RCT) (19-23). According to data, the superiority of combination therapy over TACE monotherapy remains a controversial issue. We conducted this study using comparative trials to evaluate the efficacy of combination therapy versus TACE monotherapy in unresectable HCC.
Methods
Eligibility of relevant studies
To ensure all relevant literature was covered, PubMed, EMBASE, Scopus and Cochrane Library were comprehensively searched for studies published between January 2000 and December 2017. Search terms were: (“transarterial chemoembolization” or “chemoembolization” or “TACE”) AND (“hepatocellular carcinoma” or “HCC” or “liver cancer” or “liver tumor”) AND (sorafenib). To be eligible, the studies must have explored the efficacy of combination therapy versus TACE monotherapy for unresectable HCC. References of retrieved articles were also screened.
Inclusion and exclusion criteria
Inclusion criteria
Comparative studies that explored the efficacy of sorafenib plus TACE (including conventional TACE and drug-eluting-beads TACE) versus TACE monotherapy (including TACE alone or TACE with placebo) of unresectable HCC patients were included. Studies were limited to English articles and adult patients. Necessary information, including overall survival (OS), time to progression (TTP), disease control rate (DCR), adverse events (AEs) and tumor response, should have been provided.
Exclusion criteria
Non-comparative studies or studies comparing the combination therapy versus sorafenib alone were precluded. Patients in BCLC-D were not included. Comments, editorials, letters, case reports, reviews, meta-analysis, low-level evidence and non-English literature were excluded. Studies unrelated to our topics or without useful information were also removed.
Data extraction
After the initial identification of relevant articles from the databases mentioned previously, two researchers screened the studies according to the detailed criteria by reading titles and abstracts. The number of studies in each screening procedure was recorded along with the reasons for any exclusions. Working independently, the researchers then read the full texts of the included studies and extracted the necessary information, including baseline characteristics, treatment strategy, OS, TTP, DCR, AEs and tumor response. Finally, the data was aggregated and analyzed. When disagreements occurred between the two researchers, a consensus would be reached through a discussion involving all of the researchers.
Statistical analysis
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions. Continuous variables, including OS and TTP, were presented with hazard ratio (HR) and 95% confidence interval (CI), categorical variables were as described as percentages and frequencies. The quality of included RCT studies was assessed using the Jadad scale (24), and non-RCT were adopted by methodological index for non-randomized studies (MINORS) (25). Forest plots were used to merge the weighted of effects. Ih analysis was used to assess heterogeneity among studies. If the Isvalue was less than 50%, a fixed-effect meta-analysis model would be conducted. Otherwise, a random-effects model would be established. Subgroup analysis and sensitivity analysis were performed to explain the potential source of heterogeneity. Funnel plots were used to evaluate publication bias. For all outcomes, P value <0.05 indicated statistically significant. All analyses were conducted by Revman 5.3.
Results
Identification of eligible studies
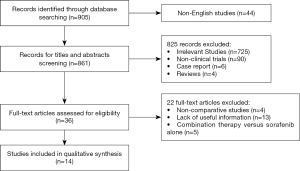
Following a search of multiple databases, a total of 905 studies were identified for initial screening. Then, according to their titles and abstracts, 869 studies were excluded, and the full texts of the remaining 36 studies were carefully examined according to inclusion and exclusion criteria. Finally, 14 comparative studies were admitted to this meta-analysis, including 4 prospectively randomized controlled trials and 10 respective studies. The flowchart of the study recruitment was shown in Figure 1.
Study characteristics
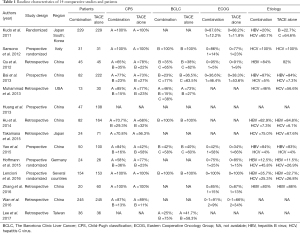
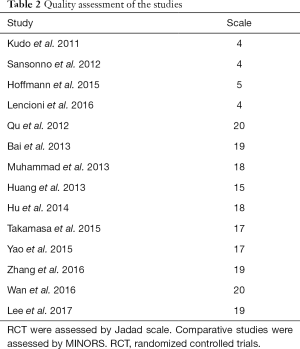
The 14 studies, which were published from 2011 to 2017, were categorized as 4 RCT and 10 non-RCT. A total of 2,602 patients were included. The sample size among the studies ranged from 13 to 245. DEB-TACE was used in 2 of the studies (22,26), and the others were conventional TACE (c-TACE). Five studies performed TACE plus sorafenib versus TACE with placebo (19-22,27). Sorafenib was initiated after TACE in 10 of the studies, with the majority starting sorafenib within a week. One study started sorafenib before TACE (22). Fifty percent patients of Kudo et al. performed sorafenib 9 weeks after TACE (19). For 8 studies which provided BCLC staging, 3 studies included all patients in BCLC-B stage (20,22,28). Nine studies described Eastern Cooperative Oncology Group (ECOG) scores, and patients in 7 studies are of 0–1. Eleven studies provided etiology of the patients and HBV was the primary reason of HCC, 1 study included only HCV patients (20) (Table 1). Quality assessment was shown in Table 2.
Full table
Full table
Treatment outcome
TTP
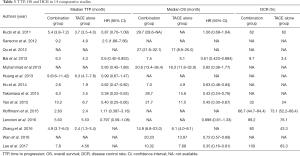
Eleven studies provided median TTP, which ranged from 2.6 to 10.2 months, and 10 studies reported the data of HR and 95% CI (Table 3). The HR for TTP in a random effect model is 0.72 (95% CI: 0.59–0.88, P=0.0010), I in a random effect model (Figure 2). Subgroup analysis was then conducted. A forest plot performed in a fixed effect model showed the HR for TTP in the RCT group was 0.84 (95% CI: 0.70–0.99, P=0.04), and the HR for TTP in a random effect model in the non-RCT group was 0.66 (95% CI: 0.49–0.90, P=0.008), indicating that combination therapy significantly prolonged TTP. Moreover, DEB-TACE showed no statistical difference for prolonging TTP when compared with c-TACE (P=0.15).
Full table

OS
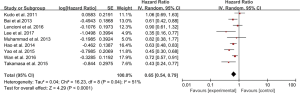
Ten studies reported that median OS ranged from 7.5 to 29.7 months. Nine studies presented HR for OS (Table 3). A forest plot concluded that the HR for OS was 0.65 (95% CI: 0.54–0.79, P<0.0001), showing that the combination therapy prolonged survival compared with TACE monotherapy. The data was performed in a random effect model and I2 for heterogeneity is 51% (Figure 3).
DCR
DCR was defined as complete response (CR), partial response (PR) and stable disease (SD). CR was defined as the absence of contrast enhancement within the original tumor. Progression disease (PD) was defined as a 25% increase in tumor size or development of a new lesion. Five studies provided DCR ranging from 30% to 89.2% (21-23,27,28). For all studies, DCR in combination group were higher than TACE alone.
AEs
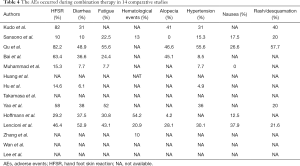
Ten studies provided AEs mainly including hand-foot skin reaction (HFSR), fatigue, diarrhea, fatigue, alopecia, hypertension and nausea (Table 4). HFSR had the highest incidence in six studies. Fatigue and diarrhea were also in high incidence. The majority of the studies graded AEs according to the Criteria for Adverse Events (CTCAE) and found most AEs were mild to moderate. Severe AEs and disease progression was the major reason for sorafenib dose adjustments. No AEs-related death and disability were presented.
Full table
Discussion
In this meta-analysis and systematic review, we have investigated 14 comparative studies, including 4 RCT trials and 10 retrospective comparative studies, to explore the effects of TACE plus sorafenib on the survival of HCC patients in comparison with those treated with TACE alone (19-31). Our study finally concluded that combination therapy of TACE plus sorafenib can not only improve TTP (HR =0.72, 95% CI: 0.59–0.88, P=0.001) but also OS (HR =0.65, 95% CI: 0.54–0.79, P<0.0001).
As the recommended therapy treated for BCLC-B HCC patients, TACE blocks the artery feeding the tumor. However, with increased embolization time and repeated application of TACE, tumor hypoxia and necrosis would result in disease progression and metastasis, which could result in HCC recurrence (32). Several studies found that sorafenib therapy extends the interval between courses of TACE, and may better preserve liver function in patients with HCC (23,24). Moreover, better liver function can not only extend the treatment’s duration but also improve the quality of life of the patient.
The first global randomized controlled study with a large sample size by Lencioni et al. has shown that sorafenib plus TACE failed to improve TTP in a clinically meaningful manner when compared with TACE mono-therapy (22). In contrast, however, later studies demonstrated that combination therapy showed superiority of survival and TTP over TACE mono-therapy (20,24,25,28). It may be because that SPACE trial had shorter treatment duration and TACE was discontinued earlier. Similarly, in the study of Kudo et al., subgroup analysis in the Korean subgroup suggested that longer sorafenib treatment duration was associated with improved TTP, in contrast with there being no difference in the Japanese subgroup, the duration of whose treatment was substantially shorter (31 versus 16 weeks) (19). This indicated that longer treatment duration makes a difference in prolonging survival outcomes and that the amount of combined treatment received may be a critical determinant of the clinical outcome.
With regard to combination therapy being preferable to treatment with TACE alone, the study by Kudo et al. had negative results (19). However, 50% patients included in this study received sorafenib 9 weeks after TACE, while most positive studies initiated sorafenib within 3–7 days (11,27,29). Thus, we inferred that the timing of post-TACE sorafenib may also have contributed to the absence of a positive effect of sorafenib.
AEs induced by sorafenib were mainly mild to moderate and could be managed by dose reduction or interruption. However, excessive drug withdrawal could result in a much lower dose than planned and hence bring negative effects on the normal functioning of drug efficacy.
In our studies, we concluded that DEB-TACE showed no statistical difference for prolonging TTP compared with c-TACE (P=0.15), which was consistent with outcome of Golfieriet et al. that the efficacy was equal between DEB-TACE and the c-TACE (33).
The major potential limitations of this study could be listed as follows. Firstly, we selected comparative studies, including both RCT and non-RCT trials, to conduct this meta-analysis. Secondly, the sample size differed greatly among different studies, and the quality of some studies was relatively lower. Thirdly, the use of different treatment options in the different studies might also influence the reliability of the conclusions. All of these factors bring potential heterogeneity to our final conclusion.
In conclusion, the combination therapy of TACE and sorafenib can significantly improve OS and TTP for unresectable HCC patients. To further support this conclusion, multicenter RCTs with large samples and good study design should be performed in future.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.115). LL serves as the unpaid editorial board member of Annals of Translational Medicine from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol 2009;24:346-53. [Crossref] [PubMed]
- Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma. Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol 2010;25:657-63. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Llovet JM, Real M, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Pesapane F, Nezami N, Patella F, et al. New concepts in embolotherapy of HCC. Med Oncol 2017;34:58. [Crossref] [PubMed]
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-9. [Crossref] [PubMed]
- Ohki T, Sato K, Yamagami M, et al. Efficacy of transcatheter arterial chemoembolization followed by sorafenib for intermediate/advanced hepatocellular carcinoma in patients in Japan: a retrospective analysis. Clin Drug Investig 2015;35:751-9. [Crossref] [PubMed]
- Shi JH, Liu SZ, Wierød L, et al. RAF-targeted therapy for hepatocellular carcinoma in the regenerating liver. J Surg Oncol 2013;107:393-401. [Crossref] [PubMed]
- Pan T, Li XS, Xie QK, et al. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol 2014;69:e553-61. [Crossref] [PubMed]
- Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer 2015;136:1458-67. [Crossref] [PubMed]
- Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008;99:2037-44. [PubMed]
- Dufour JF, Hoppe H, Heim MH, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist 2010;15:1198-204. [Crossref] [PubMed]
- Pawlik TM, Reyes DK, Cosgrove D, et al. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol 2011;29:3960-7. [Crossref] [PubMed]
- Chung YH, Han G, Yoon JH, et al. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer 2013;132:2448-58. [Crossref] [PubMed]
- Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. [Crossref] [PubMed]
- Sansonno D, Lauletta G, Russi S, et al. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist 2012;17:359-66. [Crossref] [PubMed]
- Hoffmann K, Ganten T, Gotthardtp D, et al. Impact of neo-adjuvant Sorafenib treatment on liver transplantation in HCC patients-a prospective, randomized, double-blind, phase III trial. BMC Cancer 2015;15:392. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. [Crossref] [PubMed]
- Qu XD, Chen CS, Wang JH, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer 2012;12:263. [Crossref] [PubMed]
- Yao X, Yan D, Zeng H, et al. Concurrent sorafenib therapy extends the interval to subsequent TACE for patients with unresectable hepatocellular carcinoma. J Surg Oncol 2016;113:672-7. [Crossref] [PubMed]
- Zhang YF, Wei W, Wang JH, et al. Transarterial chemoembolization combined with sorafenib for the treatment of hepatocellular carcinoma with hepatic vein tumor thrombus. Onco Targets Ther 2016;9:4239-46. [Crossref] [PubMed]
- Muhammad A, Dhamija M, Vidyarthi G, et al. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol 2013;5:364-71. [Crossref] [PubMed]
- Huang YH, Chen W, Li JP, et al. Clinical value of continuous administration of sorafenib in combination with modified transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Chin Med J (Engl) 2013;126:385-6. [PubMed]
- Hu H, Duan Z, Long X, et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS One 2013;9:e96620. [Crossref] [PubMed]
- Bai W, Wang YJ, Zhao Y, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis 2014;14:181-90. [Crossref] [PubMed]
- Wan X, Zhai X, Yan Z, et al. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget 2016;7:83806-16. [Crossref] [PubMed]
- Lee TY, Lin CC, Chen CY, et al. Combination of transcatheter arterial chemoembolization and interrupted dosing sorafenib improves patient survival in early-intermediate stage hepatocellular carcinoma: A post hoc analysis of the START trial. Medicine (Baltimore) 2017;96:e7655. [Crossref] [PubMed]
- Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21. [Crossref] [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [Crossref] [PubMed]