
Evaluation of the brain functional activities in rats various location-endometriosis pain model
Introduction
Pain is characterized typically in approximately 80% of women with endometriosis (EM), which presents nonspecific pain or hyperalgesia. These females may surfer the unendurable symptoms such as dysmenorrhoea, dyschezia, dysuria and dyspareunia detected about 78.7%, 29%, 9.9% and 44.9% respectively (1,2). Recently researches however indicated that EM-associated pain was considered as a central pain or central sensitization phenomena on account of this pain with root in amplification effect as the central nervous system. This does not imply that peripheral nociceptive input is not contributing to the pain experience (2,3). Bajaj et al. (4) first proposed that central sensitization may be involved in the occurrence and maintenance of pain related to EM. Previous studies have suggested that long-term inflammation stimulation near the local lesions leads to peripheral sensitization, which facilitates the alterations in multiple relevant intracellular signaling pathways and membrane potential between synaptic junctions, finally contributing to central sensitization (5,6). Some studies have studied the lower-location central sensitization at the spine, and found the increase of excitatory neurotransmitter transmission in dorsal rot ganglion (DRG), leading to hyperalgesia (6,7). However, the higher-position central sensitization is still relatively rare. Study of the mechanisms of pain related to the central sensitization is fundamental to our understanding and treatment of the many females with EM around the world who experienced different extent of pain.
Resting-state functional magnetic resonance imaging (rs-fMRI) is based on the contrast and change of the blood oxygenation level dependent (BOLD) under different conditions (8), which can accurately locate and dynamically observe brain functional activities and play a vital role in understanding brain structure and function. Currently, rs-fMRI has been widely used in Alzheimer’s disease (9), epilepsy (10), schizophrenia (11) and other diseases. Regional homogeneity (ReHo) analysis is widely used to measure the local connectivity activity state of a definitive voxel and its neighboring voxels around the entire brain after rs-fMRI scanning (12). The finding of Li et al. (13) indicated the ReHo value of stress rat model was increased in the thalamus, visual cortex, midbrain, cerebellum, hippocampus, hypothalamus, and olfactory cortex compared. In the field of pain, As-Sanie et al. (14) found that enhanced anterior insula glutamatergic neurotransmission and connectivity with the medial prefrontal cortex, which is the key regions of salience and default mode networks, may play a key role in the pathophysiology of the women with EM emerged chronic pelvic pain via rs-fMRI. Their study supported central pain amplification as a mechanism of chronic pelvic pain. Tian et al. (15) reported the rats with pain-related behavioral responses appeared the neuronal apoptosis and glial activation in neocortex and hippocampus via Nissl staining and Immunohistochemistry. However, the previous studies less combined the visual methods of imageology and morphology to explain the central sensitization mechanism of EM pain. Our group explored more functional activities in various brain regions using the rats different location-EM pain model through rs-fMRI, an imaging method, and the pathocytological methods to further investigate synergistically the mechanism of the higher-position central sensitization. Our finding will offer a pre-clinical experiment evidence for treatment of various location EM patients with pain.
Methods
Animal preparation
Twenty Sprague-Dawley rats weighing 201–250 g were purchased from Beijing Vital Lihua Experimental Animals Co., Ltd. (license number: SCXK 2016-0006). All rats were allowed 7 days for acclimatisation after arrival. Rats were housed and maintained (5 per cage) in a room (22±1 °C, 60–70% humidity) with a 12-hour light and dark cycle (8:00 AM to 20:00 PM) with ad libitum access to food and water. All efforts were made to minimize animal suffering. All animal process was conformed to the Ethical Inspection in Animal Experimentation, and approved by the Peking Union Medical College Hospital (Number: XHDW 2018-014).
Surgical induction of EM
All rats were divided into 4 groups of equal sizes by random number method: the first group is designated as abdominal model, second group is the gastrocnemius group, the third is ovary model and the fourth is sham surgery control, depending on the experiment design. They were surgically induced under aseptic conditions and anesthesia according to the method of Berkley (16,17). In the abdominal model (n=5 rats), the tissue of one uterine horn, approximately 5 mm in size, was removed from the rat, opened longitudinally, cut into 4 fragments. The tissue was transplanted onto the inner abdominal wall and mesentery and affixed with absorbable sutures. The equal uterine horns tissue obtained by above described method was transplanted onto the gastrocnemius muscle and ovaries, respectively in the second and third group. Two or three ectopic tissues of equal size were systematically transplanted in each rat. No transplantation in the fourth group rats, only were opened a 3-cm incision in the low abdomen as surgery control. All groups were re-opened the abdomen after two weeks from surgery to confirm successfully the models.
Pain measurement
Hotplate test (Shanghai Zhongshi Dichuong Technology Development co., Ltd. Model: zs-yls-6b, China) can be used as a measurement for the severity of pain threshold or EM-related pain due to the central sensitization (18,19). The hotplate test was performed with a commercially available hotplate meter consisting of a clear, plexiglass cylinder placed on a hotplate. They were allowed to acclimatize for 10 min before the test. All rats were brought to the plexiglass cylinder, 25 cm × 25 cm × 40 cm, allowed to walk on the hotplate (53.0±0.1 °C). The response of rats during the test was recorded test for maximum to 45 s if the animal did not appear the withdrawal of the hind paws or jump, avoiding tissue damage. The latency was calculated as the pain threshold reflecting the EM-related pain. All groups were tested from the day of operation at 4, 8 and 12 weeks respectively. Each animal was tested only once in each session.
Image acquisition
The 7.0-T little animal scanner Bruker Pharma Scan system with a 38-mm-diameter birdcage coil (Bruker BioSpin, Ettlingen, Germany, ParaVision 6.0.1) was used to scan each rat for acquiring the original data after behavioral pain assessment. The MRI data of brain anatomy image was collected using a T2-weighed sequence [repetition time (TR) =2,000 ms; field-of-view (FOV) =32×32 mm2; matrix size =256×256; slice thickness =0.7 mm; slice number =38]. The rs-fMRI images were acquired following a gradient echo-planar imaging (EPI) sequence (TR =2,000 ms; TE =600; flip angle =90°; FOV =35×35 mm2; spatial resolution =64×64; matrix size =128×128). Each rat was processed 1,200 s. All rats were anesthetized with 5% isoflurane. During the scanning, each rat was loaded into custom-made MR-compatible stereotactic holder with a bite bar to avoid head motion.
Data analysis
Rs-fMRI data were preprocessed using Statistical Parametric Mapping 12 (SPM12) software (https://www.fil.ion.ucl.ac.uk/spm/). Preprocessing steps included discarding the first 10 volumes, slice timing correction, motion correction. The rs-fMRI images were aligned to their corresponding T2-weighted images, and then normalized to the Paxinos and Watson rat brain atlas. To improve the accuracy of spatial normalization across rats, rs-fMRI data from each rat were further aligned to the group-averaged rs-fMRI images. Finally, the normalized images were linearly detrended and the six-parameter motion curves were regressed. The REST software (http://www.restfmri.sourceforge.net) was performed to calculate the ReHo value of each voxel in the whole brain, then the ReHo maps were acquired from each rat. Data were temporal band-pass filtered (0.01–0.08 Hz) to reduce the effects of low-frequency drift and physiological high-frequency. Finally, the normalized functional series were smoothed with a Gaussian kernel of 6 mm × 6 mm × 6 mm full width at half-maximum (FWHM) to improve the signal-to-noise ratio (SNR). One-way analysis of variance (ANOVA) was used to calculate significant differences in ReHo value among the four groups. The voxel level threshold was set at P<0.001 (uncorrected) and a cluster-extent threshold of 20 voxels using SPM12 software. Then the ReHo values in different brain regions were extracted for multiple comparisons by Bonferroni post hoc test using SPSS20.0 (P<0.05, correction).
Nissl staining
Nissl staining was used to observe the neurons and Nissl body in these abnormal activated brain areas located by rs-fMRI. Briefly, 5-µm-thick paraffin sections were dewaxed and rehydrated gradually, immersed in 0.5% cresyl violet for 2 min, rinsed with double distilled water, dehydrated in gradient ethanol, cleared in xylene, and sealed with neutral balsam. The Nissl staining slides were observed using a light microscope. The number of the neurons and Nissl body in 50×50 µm2 of the initial images is counted, then calculated the average number per square millimeter.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 software. The figures were performed by GraphPad Prism 7 and Adobe Illustrator CC2015. The four groups results were analyzed by ANOVA followed by Bonferroni post hoc test for multiple comparisons. The data were expressed as means ± SD and P<0.05 was considered statistically significant.
Results
Surgical induction of EM-like lesions
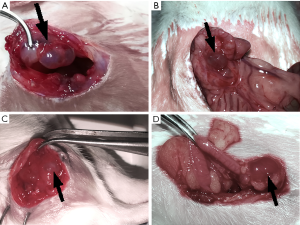
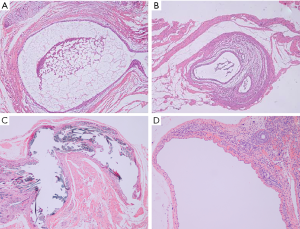
Our surgery achieved hypothetical results while all the transplant locations developed vesicular cysts at the inner side of abdominal wall, mesenterium, gastrocnemius muscle, and ovary respectively, with clear or reddish-brown fluid (as shown in Figure 1). The rats (n=4), transplant in both abdominal wall and mesentery, were divided into the abdominal EM group but outside the ovary (Figure 1A,B). The gastrocnemius group (n=5) was divided into the extra-abdominal cavity type (Figure 1C) and the ovarian group (n=5) was separately divided into another classification (Figure 1D). Finally, the sham operation group (n=5) was used as the control group. The pathological sections showed that all vesicular cysts in transplant locations were successfully induced into EM-like lesions by photon microscope. Unfortunately, a rat in the abdominal group sacrificed unexpectedly during surgery, considering anesthesia intolerance. It has been dealt with properly.
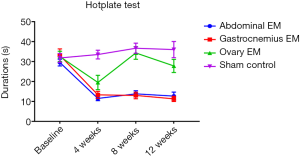
Hotplate test
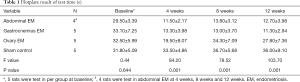
Rats with EM pain sensitization were tested by the hotplate test. It was shown there was on significantly difference in all groups (P=0.644). The duration of time prior to reaction in abdominal EM (n=4), gastrocnemius EM (n=5) and ovary EM group (n=5) was shorter than the sham group (n=5) at 4, 8, 12 weeks after surgery (Figure 2), while the abdominal EM and gastrocnemius EM was significantly shorter compared with the ovary EM group at 4, 8, 12 weeks after surgery (P=0.001) (see Table 1). There was no significantly difference in the abdominal EM and gastrocnemius EM group, which suggested EM-induced persistent pain hypersensitivity. The result indicated the EM-related pain model was confirmed by hotplate test.
Full table
Date analysis of rs-fMRI
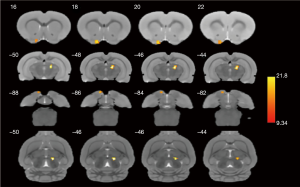
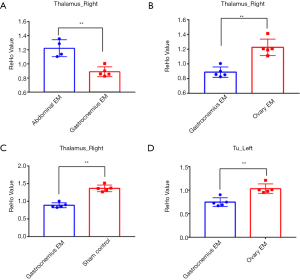
We found that there were significant differences in ReHo values among the four groups using a ANOVA. Several brain regions emerged higher ReHo signal than the whole-brain average, including right thalamus and left olfactory tubercle (P<0.001, uncorrected, see Figure 3). The more detailed information for brain regions with abnormal ReHo value in the four groups is shown in Table 2. Subsequently, the pairwise comparisons were performed to further understand which groups were different (Figure 4). In the right thalamus, there were significant differences between the abdominal EM and gastrocnemius EM group, the ReHo average values in abdominal EM group increased than gastrocnemius EM (**P<0.001, see Figure 4A). ReHo value in ovary EM also was higher than gastrocnemius EM (**P<0.001, see Figure 4B). Compared with sham control, the ReHo value was increased than gastrocnemius EM (**P<0.001, see Figure 4C). In left olfactory tubercle, the ReHo value in gastrocnemius EM decreased than ovary EM (**P<0.001, see Figure 4D). There was no significant difference between the other two-groups comparations.

Full table
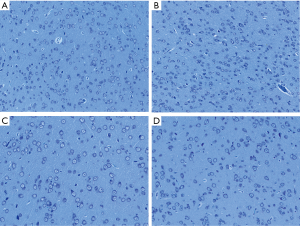
Nissl staining
Based on the observation of neurons and Nissl bodies in these abnormal activated brain areas located by rs-fMRI under Nissl staining, we found that in the right thalamus, the distribution and state of neurons and Nissl bodies were different in the groups of abdominal EM, ovary EM and sham control with higher ReHo value compared to the gastrocnemius EM group with decreased ReHo value. They presented a large number of neurons and bigger cell volume, the Nissl body was stained and the number and shape showed normal in abdominal EM, ovary EM and sham control groups (see Figure 5A,B,C). However, in the gastrocnemius EM group, the number of neurons was decreased; cavitation around nucleus, and pyknotic homogenous nuclei. Nissl bodies were stained deeply, and the shape was irregular (Figure 5D). In left olfactory tubercle, there was no significant difference in the number, volume and morphology of neurons and significant in the four groups although variety in ReHo values between them (see Figure 6A,B,C,D).

Discussion
Diverse types and locations of EM produce various degrees of pain. This study aims to further explore the central sensitization mechanism via observing whether there are differences in the brain areas of higher central nervous system to provide animal experimental evidence of pre-clinical studies for the treatment in patients with EM-related pain. Our study processed the various location-EM pain models in rats by the autotransplantation of small pieces of uterus respectively for the building of the abdominal wall and mesentery (Figure 1A,B and Figure 7A,B), which simulation is the person with EM whose lesion inside the abdominal cavity but outside the ovary according to the Berkley et al. (20,21). The rat gastrocnemius muscle model simulated some patients with EM suffering the myofascial pain and dysfunction whose endometriotic lesions located in special site outside the abdominal cavity (Figure 1C and Figure 7C) (5). The ovarian model mimicked human ovarian EM cyst according to Giamberardino et al. (Figure 1D and Figure 7D) (17). These animal models have been successfully applied to study the in vivo mechanism of EM by previous researches.
After the successful surgery, pain behavior assessment was carried out for each group of rats. Hotplate test is one of the most popular methods to evaluate pain threshold in rodents (22,23). The results of our hotplate experiment showed that the pain threshold in the abdominal EM and gastrocnemius EM was significantly lower than that in the ovarian EM group and the control group and the trend was almost consistent over time (Figure 2). The pain threshold of each group of rats was repeatedly measured at 4, 8 and 12 weeks after operation. At the 4 weeks after surgery, the pain threshold of the three EM groups was significantly lower than that of the control group, which may be because the local inflammatory response of the ectopic lesion was significant within 4 weeks after operation (2,24), and there was no difference in local pain symptoms among the three EM groups. However, the local changes such as inflammation and neovasculonerve of local lesions tend to be stable at 8, 12 weeks after the operation, the local lesions of abdominal EM group and gastrocnemius EM group have more severe inflammatory reaction and more significant pain, while the local neovasculonerve of ovarian EM group is not as obvious as that of the first two groups, hence the pain threshold is also relatively higher. These results are consistent with Iuvone et al. (25) and Zhao et al. (26). This condition is also similar to the phenomenon that pain in patients with deep EM is more obvious than that in patients with ovarian EM.
Why did the pain in the abdominal EM and gastrocnemius EM group decrease compared to the ovarian EM and control group? In addition to the stimulation theory of local neovascularization and other factors, we also think that it is related to some functional regions of the high brain central system after pain sensitization. Hence, the four groups of rats were tested by rs-fMRI. The purpose of this examination was to investigate whether the causes of different pain levels in various location-EM rats were related to functional changes in some brain regions by ReHo analysis. ReHo analysis is widely used to measure the local connectivity activity state of a definitive voxel and its neighboring voxels, and is a visual method to explore the coordination of spontaneous brain functional activity in schizophrenia, Parkinson’s disease, bipolar disorder (27-29). However, the ReHo analysis is less applied to pain research, especially in EM-related pain. Fortunately, our results supported our hypothesis. As shown in Figure 3, we conducted ReHo index on four groups of rats scanned by fMRI. Our results showed that there were significant differences in ReHo values in the right thalamic region and the left hydrangea region in the four groups of rats. To further understand which groups are different, four groups of ReHo values were extracted and pairwise compared. ReHo values decreased in gastrocnemius EM group, increased in abdominal EM, ovarian EM and control group in right thalamus and left olfactory tubercle (see Figure 4). However, what was surprising to us was that there was a significant difference in the ReHo values in the thalamic region between the Abdominal EM group with significant pain and the gastrocnemius EM by behavioral evaluation, while there was no significant difference between the abdominal EM, ovarian EM and control group (figure only lists the results with differences between pairwise comparisons). Thalamus is the most important sensory conduction replacement station. A cursory analysis and synthesis of sensation is performed in the thalamus. Yoshino et al. (30) also found the ReHo values in the thalamus of patients with chronic pain (somatoform pain disorder) significantly decreased.
Hence, in order to further understand the reasons for the differences between these abnormal brain regions, Nissl staining was performed on this brain region. Observing the state of neurons may explain this phenomenon since cells in the thalamus nucleus are sensitive to invasive afferent impulses. The result of Nissl staining may answer our doubts. As shown in Figures 5,6, the number of neurons in the thalamus of the gastrocnemius EM group was relatively reduced, the number of neurons was decreased; cavitation around nucleus, and pyknotic homogenous nuclei. Nissl bodies were stained deeply, and the morphology was deformed, in which pathological changes may occur. The other three groups had more neurons than the gastrocnemius EM group, the morphology of Nissl body was regular. There was no difference among the three groups. Oxidative stress reaction is enhanced when central nervous system is subjected to external stimuli (31). Neuron is the carrier of redox reaction, and the brain region with more neurons may have more vigorous oxygen activity (32). Excessive oxidative stress can cause the function or structure of the nerve cells abnormalities, such changes become are detected by fMRI, Nissl staining results are also verified the ReHo analysis in our study, namely significantly different pain in EM rats, increased the number of nerve cells, increase the cell body, Nissl body increase, morphological diversity. It is known that the olfactory tubercle was not associated with pain, but the results showed abnormal activation (higher ReHo value) of this brain region, which may have other factors unknown to us, or may be a false positive result. Nissl staining showed no difference in the state and number of neurons and Nissl body in this brain region among the four groups. Therefore, we need to expand the sample size to continue to explain this phenomenon for enhancing the reliability of the experiment.
Acknowledgments
The authors thank the rats made sacrifices for the medical experiment, and all the staff who participated in this experiment.
Funding: This work was supported by the grant of the National Natural Science Foundation of China (grant number: 81471440), the National Key Research and Development Program of China (grant number: 2017YFC1001200) and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (grant number: CAMS-2017-I2M-1-002).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal process was conformed to the Ethical Inspection in Animal Experimentation, and approved by the Peking Union Medical College Hospital (Number: XHDW 2018-014).
References
- Sinaii N, Plumb K, Cotton L, et al. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril 2008;89:538-45. [Crossref] [PubMed]
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol 2018;51:53-67. [Crossref] [PubMed]
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547-55. [Crossref] [PubMed]
- Bajaj P, Bajaj P, Madsen H, et al. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain 2003;4:372-80. [Crossref] [PubMed]
- Aredo JV, Heyrana KJ, Karp BI, et al. Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin Reprod Med 2017;35:88-97. [Crossref] [PubMed]
- Kuner R. Central mechanisms of pathological pain. Nat Med 2010;16:1258-66. [Crossref] [PubMed]
- Kryzhanovskiĭ GN. Central nervous system mechanisms of pathological pain. Zh Nevrol Psikhiatr Im S S Korsakova 1999;99:4-7. [PubMed]
- Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868-72. [Crossref] [PubMed]
- Mascali D, DiNuzzo M, Serra L, et al. Disruption of Semantic Network in Mild Alzheimer's Disease Revealed by Resting-State fMRI. Neuroscience 2018;371:38-48. [Crossref] [PubMed]
- Omidvarnia A, Kowalczyk MA, Pedersen M, et al. Towards fast and reliable simultaneous EEG-fMRI analysis of epilepsy with automatic spike detection. Clin Neurophysiol 2019;130:368-78. [Crossref] [PubMed]
- Ramkiran S, Sharma A, Rao NP. Resting-state anticorrelated networks in Schizophrenia. Psychiatry Res Neuroimaging 2019;284:1-8. [Crossref] [PubMed]
- Pang R, Guo R, Wu X, et al. Altered Regional Homogeneity in Chronic Insomnia Disorder with or without Cognitive Impairment. AJNR Am J Neuroradiol 2018;39:742-7. [Crossref] [PubMed]
- Li J, Yang R, Xia K, et al. Effects of stress on behavior and resting-state fMRI in rats and evaluation of Telmisartan therapy in a stress-induced depression model. BMC Psychiatry 2018;18:337. [Crossref] [PubMed]
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional Connectivity is Associated With Altered Brain Chemistry in Women With Endometriosis-Associated Chronic Pelvic Pain. J Pain 2016;17:1-13. [Crossref] [PubMed]
- Tian GH, Tao SS, Chen MT, et al. Electroacupuncture Treatment Alleviates Central Poststroke Pain by Inhibiting Brain Neuronal Apoptosis and Aberrant Astrocyte Activation. Neural Plast 2016;2016:1437148. [PubMed]
- Berkley KJ, Cason A, Jacobs H, et al. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett 2001;306:185-8. [Crossref] [PubMed]
- Giamberardino MA, Berkley KJ, Affaitati G, et al. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain 2002;95:247-57. [Crossref] [PubMed]
- Liu X, Yan D, Guo SW. Sensory nerve-derived neuropeptides accelerate the development and fibrogenesis of endometriosis. Hum Reprod 2019;34:452-68. [Crossref] [PubMed]
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci 2007;Chapter 8:Unit 8.9.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science 2005;308:1587-9. [Crossref] [PubMed]
- Berkley KJ, Dmitrieva N, Curtis KS, et al. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A 2004;101:11094-8. [Crossref] [PubMed]
- Tommasi A, Cocuzza M, Perrone D, et al. Modeling, Fabrication and Testing of a Customizable Micromachined Hotplate for Sensor Applications. Sensors (Basel) 2016. [Crossref] [PubMed]
- Chauhan VM, Hopper RH, Ali SZ, et al. Thermo-optical characterization of fluorescent rhodamine B based temperature-sensitive nanosensors using a CMOS MEMS micro-hotplate. Sens Actuators B Chem 2014;192:126-33. [Crossref] [PubMed]
- Paul Dmowski W, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol 2004;18:245-63. [Crossref] [PubMed]
- Iuvone T, Affaitati G, De Filippis D, et al. Ultramicronized palmitoylethanolamide reduces viscerovisceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis: role of mast cells. Pain 2016;157:80-91. [Crossref] [PubMed]
- Zhao T, Liu X, Zhen X, et al. Levo-tetrahydropalmatine retards the growth of ectopic endometrial implants and alleviates generalized hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci 2011;18:28-45. [Crossref] [PubMed]
- Yang X, Luo J, Zhong Z, et al. Abnormal Regional Homogeneity in Patients With Obsessive-Compulsive Disorder and Their Unaffected Siblings: A Resting-State fMRI Study. Front Psychiatry 2019;10:452. [Crossref] [PubMed]
- Shao Y, Li QH, Li B, et al. Altered brain activity in patients with strabismus and amblyopia detected by analysis of regional homogeneity: A restingstate functional magnetic resonance imaging study. Mol Med Rep 2019;19:4832-40. [PubMed]
- Hu J, Xiao C, Gong D, et al. Regional homogeneity analysis of major Parkinson's disease subtypes based on functional magnetic resonance imaging. Neurosci Lett 2019;706:81-7. [Crossref] [PubMed]
- Yoshino A, Okamoto Y, Doi M, et al. Regional brain functions in the resting state indicative of potential differences between depression and chronic pain. Sci Rep 2017;7:3003. [Crossref] [PubMed]
- Pei JP, Fan LH, Nan K, et al. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J Neuroinflammation 2017;14:97. [Crossref] [PubMed]
- Lee JY, Maeng S, Kang SR, et al. Valproic Acid Protects Motor Neuron Death by Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress-Mediated Cytochrome C Release after Spinal Cord Injury. J Neurotrauma 2014;31:582-94. [Crossref] [PubMed]


