
Impact of squamous differentiation on intravesical recurrence and prognosis of patients with upper tract urothelial carcinoma
Introduction
Urothelial carcinoma arising from the course of urothelium between urethra and renal pelvis has been one of commonly diagnosed neoplasms (1). As urothelial malignancy, upper urinary tract urothelial carcinoma (UTUC) was comprised of ureteral and renal pelvis carcinoma. Compared with bladder cancer, UTUC is relatively rare and accounts for only 5% of all urothelial carcinoma, but it is often aggressive with poor prognosis (2,3). The majority of UTUCs are pure urothelial carcinomas, however, different histological variants of UTUC have been pronounced in approximately 40% of UTUC cases (4). Squamous differentiation is the most common pathologic variant of urothelial carcinoma (4,5). Well established though the prognostic significance of squamous differentiation in bladder urothelial carcinoma has been, the clinical impact of squamous differentiation in UTUC is still conflicted (5-14). Therefore, we are devoted to investigating the clinicopathological and prognostic significance of squamous differentiation in UTUC.
Methods
Patients
The present study was managed upon approval from the Institutional Review Board of the Second Hospital of Tianjin Medical University. A sum of 770 patients who underwent radical nephroureterectomy (RNU) for UTUC in the hospital, during the period of April 2003 to April 2016, were selected for this retrospective analysis. The inclusion criteria of clinical disease involved pathological stage Ta–T4 and complete surgical resection without positive margins. Patients with a history of bladder cancer were excluded because treatment for bladder cancer may affect the histology. Involved patients were confirmed without bladder urothelial carcinoma via cystoscope before surgery. Afterwards, 669 pure UTUC patients along with 101 UTUC patients with squamous differentiation, without distant metastasis, made up the current study cohorts. None of the above patients received neoadjuvant chemotherapy and experienced confirmed lymph node metastasis before surgery. Patients received intravesical chemotherapy with Pirarubicin or Camptothecins as essential treatments after RNU for half to full year. Systemic chemotherapy was performed on patients with lymphatic metastasis after surgery. Moreover, patients with suspicious enlarged lymph nodes on preoperative radiology or with intraoperatively abnormal observations received regional lymphadenectomy. The extent and number of lymphadenectomies performed were determined by the surgeons. Clinical and pathological information were retrieved from patients’ charts and electronic medical records. Parameters including age, gender, side of tumor, primary site, tumor grade, tumor stage, lymphovascular invasion and intravesical recurrence were recorded.
Pathology
All RNU specimens were processed by genitourinary pathologists at our hospital. Each of the pathology slides was evaluated case by case methodically and all slides were re-determined by another senior pathologist. The pathologic stage was determined according to the 2010 American Joint Committee on Cancer TNM staging system (15). Pathologic grading of each tumor was considered under the 2004 World Health Organization grading system. Tumor location was defined as renal pelvis, ureter or both of them based on dominant tumor features (16). The assessment of squamous differentiation, with keratinization or intercellular bridges serving as indicator, was carried out by hematoxylin and eosin (H&E) staining. H&E staining was applied to record the lymphovascular invasion. No changes in the criteria for determining characterization of squamous differentiation occurred all along the whole study.
Statistical methods
Cancer specific survival calculated the time gap from the date of surgery to the date of UTUC specific death. The independent-sample Student’s t-test and chi-square test were applied to make evaluation on continuous variables and categorical variables, respectively. The log-rank test was devoted to examining distinction while the Kaplan-Meier curve was carried to describe overall survival trends and curves. Landmark survival analysis was also used to determine cancer specific survival with Empower Stats software. Univariate analyses using the Cox regression models were performed to examine the cancer specific survival after operation and only the significant factors were entered into multivariate models. A p value (no more than 5 percent, two-sided) was considered to present a statistically significant difference, and hazard ratios with 95% CIs from the Cox model were used. The whole statistical analysis was performed on SPSS 22 statistical software (SPSS, IBM Corporation, Armonk, NY, USA).
Results
Clinical characteristics
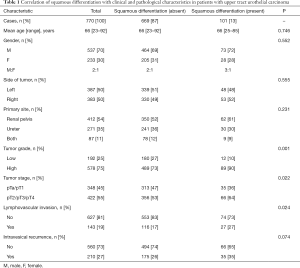
Clinical and pathological characteristics were presented in Table 1. Patients were 66 years old on average, and 537 male patients and 233 female patients were included in this study, with male-to-female ratio of 2:1. Among the 770 patients, squamous differentiation was determined in 101 (13%) patients. Squamous differentiation was not significantly associated with age (P=0.746), gender (P=0.552), side of tumors (P=0.555), primary site (P=0.231) and intravesical recurrence (P=0.074). However, higher percentage of occurrence was tested on high grade tumors with squamous differentiation, compared to those without squamous differentiation (90% vs. 73%, P=0.001). Patients in UTUC with squamous differentiation group showed a higher frequency of more advanced disease (pT2/pT3/pT4) (P=0.022) in comparison with the pure UTUC group. Dramatic difference of lymphovascular invasion rates were captured in the above two cohorts, lymphovascular invasion was more common to be noted on UTUC with squamous differentiation [27 squamous differentiation-present patients (27%) vs. 116 squamous differentiation-absent patients (17%)] (P=0.024). Intravesical recurrent patients with squamous differentiation was 35 (35%) and intravesical recurrent patients without squamous differentiation was 175 (26%) (P=0.074). Namely, there were no significant links between squamous differentiation and the occurrence of intravesical recurrence in UTUC. The quadripartite patients, stratified by tumor stage and grade, were listed in Table 2. Surprisingly, in early stage (pTa/pT1) UTUC patients, high-grade tumors did not prevail in squamous differentiation-present cohort [29 squamous differentiation-present patients (83%) vs. 225 squamous differentiation-absent patients (72%)] (P=0.166). However, UTUC patients with squamous differentiation showed a higher frequency of high-grade disease (P=0.003) in advanced stage.
Full table

Full table
Oncological outcome
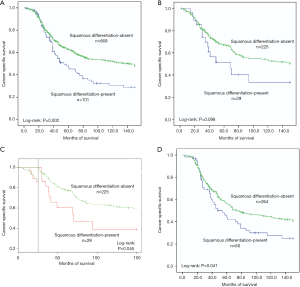
In this retrospective study, the median follow-up was 50 months, with the minimum of 2 and maximum of 150. Suffering from cancer metastasis, 350 patients (45%) died in this follow-up period. The overall 5-year cancer specific survival rate was 61%. To be more specific, the 5-year cancer specific survival rates were 47% for squamous differentiation-present patients while 63% for squamous differentiation-absent patients (P=0.002, Figure 1A). In subgroup of early-stage (pTa/pT1), only 6 low-grade patients presented squamous differentiation (Table 2). Because of great statistical bias for survival analysis, we did not investigate the impact of squamous differentiation on low-grade patients in early stage cohort. Among high-grade patients in early stage group, the 5-year cancer specific survival rate was 67% in squamous differentiation-absent patients and 52% in squamous differentiation-present patients (P=0.098, Figure 1B). According to Kaplan-Meier curve in Figure 1B, we found significant survival differences between squamous differentiation positive and negative patients after 25–30 months’ survival. Then, we estimated cancer specific survival of these patients using Landmark survival analysis methods with time from the 25-month landmark. The results showed that high-grade patients with squamous differentiation tended to have a significant decrease of cancer specific survival after previous 25-month survival in early stage (P=0.045, Figure 1C). In subgroup of advanced pathologic T stage (pT2/pT3/pT4), the few low-grade patients with squamous differentiation was not suitable for analysis of survival (Table 2). Meanwhile, in advanced stage group, the 5-year cancer specific survival rates were 57% in squamous differentiation-absent high-grade patients and 44% in squamous differentiation-present high-grade patients (P=0.041, Figure 1D).
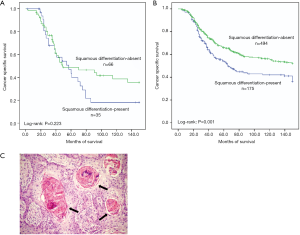
Intravesical recurrence was observed in 210 (27%) patients. However, significant differential frequency of intravesical recurrence was not detected between squamous differentiation positive and negative patients (35% vs. 26%, P=0.074) (Table 1). We also found that intravesical recurrence had little influence on the cancer specific survival of squamous differentiation-present patients (P=0.223, Figure 2A). On the contrary, intravesical recurrence tended to decrease cancer specific survival among squamous differentiation-absent patients (P=0.001, Figure 2B).
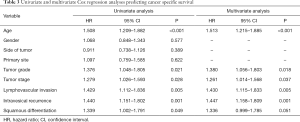
Cox proportional hazard analysis was applied for further analysis (Table 3). In the results of univariate analysis, age (HR 1.508, 95% CI: 1.209–1.882, P<0.001), tumor grade (HR 1.376, 95% CI: 1.048–1.805, P=0.021), tumor stage (HR 1.279, 95% CI: 1.026–1.593, P=0.028), lymphovascular invasion (HR 1.429, 95% CI: 1.112–1.836, P=0.005), intravesical recurrence (HR 1.440, 95% CI: 1.151–1.802, P=0.001) and squamous differentiation (HR 1.339, 95% CI: 1.002–1.791, P=0.049) were examined to be the prognostic factors correlated with cancer specific survival rates. According to the results of the multivariate Cox proportional hazard analysis, age (HR 1.513, 95% CI: 1.215–1.885, P<0.001), tumor grade (HR 1.380, 95% CI: 1.056–1.803, P=0.018), tumor stage (HR 1.261, 95% CI: 1.014–1.568, P=0.037), lymphovascular invasion (HR 1.430, 95% CI: 1.115–1.833, P=0.005), and intravesical recurrence (HR 1.447, 95% CI: 1.158–1.809, P=0.001) significantly influenced the cancer specific survival rates. However, in multivariate analyses adjusted for the tumor grade and other clinicopathological factors, there were no significant associations between squamous differentiation and patients’ outcomes. That is to say, the squamous differentiation did not remain as a significant independent predictor of cancer specific survival (HR 1.336, 95% CI: 0.999–1.785, P=0.051).
Full table
Pathology characteristics
Overall 101 squamous differentiation patients were diagnosed and confirmed by pathologists. The occurrence of squamous differentiation was confirmed with a sufficient indicator of intercellular bridges and/or keratinization (Figure 2C). The major histological characteristic includes: (I) strands or nests of infiltrating tumor cells contains large and medium sized nucleolus; (II) no clearly separated amphophilic or eosinophilic cytoplasmic background.
Discussion
Compared with bladder cancer, UTUC tends to have worse survival and prognosis according to tumor characteristics (17,18). Although previous studies had shown the significant prognostic role of squamous differentiation in UTUC, results about the impact of squamous differentiation on patients’ survival were still inconsistent among these reports (19-22). Makise et al. retrospectively analyzed the hematoxylin and eosin stained slides and clinical records of 140 UTUC patients, and found that squamous differentiation was more likely to shorten the cancer specific survival. But the authors suggested that squamous differentiation was not an independent predictor of worse prognosis for UTUC patients (23). Lee et al. evaluated the clinicopathologic data of 696 UTUC patients treated with RNU and shown that patients with squamous differentiation in UTUC tended to have poorer cancer specific survival and overall survival (20). Multivariate Cox regression analysis from this paper revealed that squamous differentiation was an independent predictor for cancer specific survival (20). Kim et al. retrospectively selected 452 patients who received RNU for UTUC and determined the effect of variant histology on the patients (24). They confirmed that the cancer specific survival of patients with squamous differentiation descended significantly while compared with the pure UTUC patients and variant histology as an independent prognostic factor harbored aggressive tumor biological characteristics (24).
The current study proposed that squamous differentiation in UTUC was correlated with clinicopathological features and clinical outcome. Compared with pure UTUC, high grade (90% vs. 73%, P=0.001), advanced stage (pT2/pT3/pT4) (64% vs. 53%, P=0.022), and lymphovascular invasion (27% vs. 17%, P=0.024) were significantly associated with squamous differentiation. Detection of intravesical recurrence was also greater in the cohort of patients with squamous differentiation (35% vs. 26%), but no significant influence was observed (P=0.074). For early stage patients, squamous differentiation was not significantly associated with tumor grade. However, early stage patients with squamous differentiation tend to receive high tumor grade (83% vs. 72%). The occurrence of squamous differentiation significantly correlated with the presence of high grade in advanced stage patients. Our results revealed that age (HR 1.508, 95% CI: 1.209–1.882, P<0.001), tumor grade (HR 1.376, 95% CI: 1.048–1.805, P=0.021), tumor stage (HR 1.279, 95% CI: 1.026–1.593, P=0.028), lymphovascular invasion (HR 1.429, 95% CI: 1.112–1.836, P=0.005), intravesical recurrence (HR 1.440, 95% CI: 1.151–1.802, P=0.001) and squamous differentiation (HR 1.339, 95% CI: 1.002–1.791, P=0.049) were significantly associated with poorer prognosis in univariate Cox proportional hazard analysis. However, presence of squamous differentiation lacked independent influence on patient outcome in multivariate Cox analysis. The present results suggested that squamous differentiation was correlated with tumor progression, but was not an independent predictor of worse prognosis in UTUC patients. Although several previous studies proposed the independent prognostic in UTUC (19,20,22), other reports and our study failed to confirm that (5,21). Several factors like group classification, methods of pathological examination, and covariates included in the multivariate model attributed the inconsistent conclusions. Classification of squamous and glandular differentiation into one group as a major reason accounts for the independent prognostic role of squamous differentiation in some studies without accurate description of solely squamous differentiation prognostic effect (20).
In order to determine the impact of squamous differentiation on cancer specific survival accurately, we compared the cancer specific survival between squamous differentiation-present and squamous differentiation-absent cohorts based on similar stage patients. The few squamous differentiation-present low-grade patients limited the statistics of survival analysis, and more patients need to be enrolled in the future. Our present findings showed that high-grade squamous differentiation-present patients had poorer 5-year cancer specific survival rates than high-grade squamous differentiation-absent patients with same tumor stage UTUC. Namely, high-grade UTUC patients ought to be concerned about the aggressive role of squamous differentiation after RNU. Among high-grade UTUC patients in early stage, squamous differentiation more likely to induced worse prognostic outcome after 25 years.
Although our series suggested squamous differentiation was not associated with intravesical recurrence significantly, patients with squamous differentiation tend to obtain high risk of intravesical recurrence (35% vs. 26%). Worse prognostic influence of intravesical recurrence in squamous differentiation-absent patients did not occurred in squamous differentiation-present patients. That is to say intravesical recurrence was not an independent prognostic factor for squamous differentiation-present patients based on similar cancer specific survival (P=0.223).
In our study, no patients received neoadjuvant chemotherapy and experienced confirmed lymph node metastasis before surgery. Intravesical chemotherapy with Pirarubicin or Camptothecins was performed routinely on UTUC patients after RNU, in accordance with bladder urothelial carcinoma. However, a single post-operative dose of intravesical chemotherapy (mitomycin C, pirarubicin) immediately after surgery was recommended based on the European Association of Urology Guidelines 2017. If lymphatic metastasis was detected in patients, systemic chemotherapy cannot be avoided after surgery. In addition, patients with suspicious enlarged lymph nodes on preoperative radiology or with intraoperatively abnormal observations received regional lymphadenectomy. Current guidelines recommend high risk UTUC should proceed Lymphadenectomy and extra-nodal extension, but previous years the extent and number of lymphadenectomies performed were determined by the surgeons. Patients treated in single center provided consistent evaluation, same treatment approach on all the subjects, and follow up, which was appropriate to evaluate outcomes.
There are a few limitations in present study. Our data were retrospective in nature and inherent biases occurred in a patient selection and treatment. The pathological and clinical data was collected from a single institution and the size of patients was not large enough. It should be noted that the size of low-grade UTUC patients with squamous differentiation was small, and thus, limitations occurred. However, these limitations are unavoidable, taking the deficiency of UTUC with squamous differentiation into consideration. Further studies involving larger scale of patients are still needed to value the significant role of squamous differentiation.
Conclusions
As it is concerned, squamous differentiation in UTUC was associated with advanced stage and higher grade, when compared to pure UTUC. In the present study, the presence of squamous differentiation was a vital prognostic factor for cancer specific survival and correlated with intravesical recurrence after receiving RNU.
Acknowledgments
Medbanks (Beijing) Network Technology Co., Ltd was thanked for data collection from patients’ charts and electronic medical records.
Funding: The whole process of our study was supported by Tianjin Municipal Natural Science Foundation (Grant 17JCYBJC26000), Nature Science Foundation of Tianjin (Grant 15JCZDJC35400), Medical and Health Science and Technology Development Project of Shandong Province (Grant 2017WS687), Postgraduate Innovation Fund of “13th Five-Year Comprehensive Investment”, and Tianjin Medical University (YJSCX201810). These funding sources have no implication in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was approved by the independent ethical committee/institutional review board of the Second Hospital of Tianjin Medical University, Tianjin Institute of Urology, Tianjin, China (ID:KY2019K031) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Oosterlinck W, Solsona E, van der Meijden AP, et al. EAU guidelines on diagnosis and treatment of upper urinary tract transitional cell carcinoma. Eur Urol 2004;46:147-54. [Crossref] [PubMed]
- Park S, Hong B, Kim CS, et al. The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol 2004;171:621-5. [Crossref] [PubMed]
- Perez-Montiel D, Wakely PE, Hes O, et al. High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol 2006;19:494-503. [Crossref] [PubMed]
- Rink M, Robinson BD, Green DA, et al. Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J Urol 2012;188:398-404. [Crossref] [PubMed]
- Mitra AP, Bartsch CC, Bartsch G Jr, et al. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol 2014;32:117-27. [Crossref] [PubMed]
- Mazzucchelli L, Bacchi M, Studer UE, et al. Invasion depth is the most important prognostic factor for transitional-cell carcinoma in a prospective trial of radical cystectomy and adjuvant chemotherapy. Int J Cancer 1994;57:15-20. [Crossref] [PubMed]
- Yang MH, Yen CC, Chen PM, et al. Prognostic-factors-based risk-stratification model for invasive urothelial carcinoma of the urinary bladder in Taiwan. Urology 2002;59:232-8; discussion 238-9. [Crossref] [PubMed]
- Antunes AA, Nesrallah LJ, Dall'Oglio MF, et al. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol 2007;33:339-45; discussion 346. [Crossref] [PubMed]
- Erdemir F, Tunc M, Ozcan F, et al. The effect of squamous and/or glandular differentiation on recurrence, progression and survival in urothelial carcinoma of bladder. Int Urol Nephrol 2007;39:803-7. [Crossref] [PubMed]
- Frazier HA, Robertson JE, Dodge RK, et al. The value of pathologic factors in predicting cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer 1993;71:3993-4001. [Crossref] [PubMed]
- Honma I, Masumori N, Sato E, et al. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology 2004;64:744-8. [Crossref] [PubMed]
- Jozwicki W, Domaniewski J, Skok Z, et al. Usefulness of histologic homogeneity estimation of muscle-invasive urinary bladder cancer in an individual prognosis: a mapping study. Urology 2005;66:1122-6. [Crossref] [PubMed]
- Kim SP, Frank I, Cheville JC, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol 2012;188:405-9. [Crossref] [PubMed]
- Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg 2002;87:13-5. [PubMed]
- Lee HY, Li CC, Huang CN, et al. Prognostic significance of lymphovascular invasion in upper urinary tract urothelial carcinoma is influenced by tumor location. Ann Surg Oncol 2015;22:1392-400. [Crossref] [PubMed]
- Gandaglia G, Bianchi M, Trinh QD, et al. Survival after nephroureterectomy for upper tract urothelial carcinoma: a population-based competing-risks analysis. Int J Urol 2014;21:249-56. [Crossref] [PubMed]
- Raman JD, Messer J, Sielatycki JA, et al. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int 2011;107:1059-64. [Crossref] [PubMed]
- Jang NY, Kim IA, Byun SS, et al. Patterns of failure and prognostic factors for locoregional recurrence after radical surgery in upper urinary tract transitional cell carcinoma: implications for adjuvant radiotherapy. Urol Int 2013;90:202-6. [Crossref] [PubMed]
- Lee YJ, Moon KC, Jeong CW, et al. Impact of squamous and glandular differentiation on oncologic outcomes in upper and lower tract urothelial carcinoma. PLoS One 2014;9:e107027. [Crossref] [PubMed]
- Langner C, Hutterer G, Chromecki T, et al. Patterns of invasion and histological growth as prognostic indicators in urothelial carcinoma of the upper urinary tract. Virchows Arch 2006;448:604-11. [Crossref] [PubMed]
- Kim DS, Lee YH, Cho KS, et al. Lymphovascular invasion and pT stage are prognostic factors in patients treated with radical nephroureterectomy for localized upper urinary tract transitional cell carcinoma. Urology 2010;75:328-32. [Crossref] [PubMed]
- Makise N, Morikawa T, Kawai T, et al. Squamous differentiation and prognosis in upper urinary tract urothelial carcinoma. Int J Clin Exp Pathol 2015;8:7203-9. [PubMed]
- Kim JK, Moon KC, Jeong CW, et al. Variant histology as a significant predictor of survival after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Urol Oncol 2017;35:458.e9-e15. [Crossref] [PubMed]


