Screening and combining serum biomarkers to improve their diagnostic performance in the detection of intestinal barrier dysfunction in patients after major abdominal surgery
Introduction
Intestinal mucosal barrier dysfunction is a common complication after major surgery, severe trauma and critical illness. When it occurs, intestinal mucosal permeability can be increased. As a result, intestinal bacteria, endotoxins and other harmful substances escape the intestinal tissue, triggering a series of pathophysiological changes and even systemic inflammatory response syndrome and multiple organ dysfunction syndrome (1-4).
Early diagnosis and effective measures can effectively improve the prognosis of patients. However, effective detection methods for intestinal barrier dysfunction are currently lacking. Serum biomarkers have recently attracted the attention of researchers because biomarkers reflecting intestinal mucosal barrier damage from different angles have been identified in some clinical and animal studies. For example, A-glutathione-S-transferase (α-GST) is a highly active structural enzyme in intestinal mucosal epithelial cells (5). D-lactic acid is produced by metabolic processes or the cleavage of intestinal bacteria and then enters the blood through the intestinal barrier (6,7). Intestinal fatty acid-binding protein (I-FABP) is located in the necrotic top cells of the intestinal villi due to intestinal ischaemia. I-FABP is released into the blood through the portal vein and the chyle canal (8). Diamine oxidase (DAO) is a type of deamination catalytic substance that oxidizes intracellular enzymes. Greater than 95% of DAO is located in the epithelial cells of the small intestine and can decompose polyamines, such as histamine, and control the proliferation of mucosa. Citrulline is mainly synthesized by glutamine as a precursor substance in the intestinal mucosa. When the epithelial cells of the intestinal mucosa are damaged, the synthesis of citrulline is significantly reduced (9-13). A large number of clinical and animal experiments have demonstrated that the above serum biomarkers were effective in the evaluation of intestinal ischaemia but not intestinal barrier dysfunction in intestinal ischaemic diseases, severe trauma, burns, infections or other serious stress responses (14-22).
Only a few studies have used these biomarkers to identify intestinal barrier dysfunction (23-26). However, these studies have some deficiencies in their design, such as a lack of reporting of diagnostic standards of intestinal barrier dysfunction (23), lack of diagnostic accuracy of the biomarker available (24,26), and lack of consideration of the effect of nutrients on intestinal barrier dysfunction (23-26). Therefore, a prospective well-designed clinical study is needed to evaluate the diagnostic performance of biomarkers in detecting intestinal barrier dysfunction.
In this exploratory and prospective study, we wanted to evaluate the diagnostic performance of biomarkers assessing intestinal barrier dysfunction in major abdominal surgery and to screen and combine highly effective biomarkers that reflect changes in the intestinal mucosal barrier to improve their diagnostic performance in identifying intestinal barrier dysfunction after major abdominal surgery.
Methods
Study design
This prospective, non-interventional study was conducted in the Department of Gastrointestinal Surgery, Second Affiliated Hospital of Kunming Medical University in Kunming, China, from January 2016 to December 2016. This study adhered to the principles of the Declaration of Helsinki and international ethical guidelines for human biomedical research from the Council for International Organizations of Medical Sciences (CIOMS). This study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University. All enrolled subjects read information on and understood the purpose of this study and then provided informed consent.
Study design and sample collection
Inclusion criteria were as follows: major abdominal surgery that was suspected to lead to intestinal barrier dysfunction; consecutive patients aged between 18 and 75 years; and body mass index (BMI) of 20–28 kg/m2 (27,28).
Exclusion criteria were as follows: all subjects with chronic inflammatory diseases, such as inflammatory bowel disease, chronic hepatitis, chronic pancreatitis, chronic nephritis, chronic renal failure, chronic gastrointestinal ulcers, or intestinal obstruction. Subjects were also excluded based on the use of medication, such as antibiotics, hormones, sedatives, and non-steroidal anti-inflammatory drugs, within the past 2 weeks.
Diagnostic standard of intestinal barrier dysfunction
According to the clinical diagnosis and treatment recommendations by the Digestive Disease Branch of Chinese Medical Association in 2006 (26,29), the following five diagnostic bases for intestinal barrier dysfunction were suggested:
- The patient has a critical disease that may cause intestinal barrier dysfunction.
- On the basis of the primary disease, the patient reported abdominal pain, abdominal distension, diarrhoea, constipation, gastrointestinal bleeding, food intolerance or other symptoms. The bowel sounds are weakened or have disappeared (bowel sound changes induced by anaesthesia and drugs were excluded).
- An increase in the plasma endotoxin level is observed.
- A permeability increase or low intestinal perfusion is observed.
- Bacterial culture is positive for blood or ascites, and no other clear infective lesions were noted.
Given (I) +(II) for the diagnosis of the necessary conditions, (I) +(II) +(III) +(IV) or (I) +(II) +(V) can generally be diagnosed.
In this study, (I) + (II) +(V) were used as the diagnostic criteria for intestinal barrier dysfunction. The clinical intestinal barrier dysfunction could be diagnosed by observing the clinical symptoms and the relevant results within 1 week after surgery.
O’Boyle et al. (30,31) considered that the more effective method of assessing intestinal bacterial translocation is PCR, and the most common bacteria involved in intestinal bacterial translocation are Escherichia coli (E. coli), accounting for 54% of all identified microbes. Thus, in this study, item (5) was improved, and the bacterial culture method was improved to enable better quantification of E. coli 16S rRNA by qPCR ( 32). Intestinal barrier function status is assessed by observing the patients’ clinical characteristics, gastrointestinal function and E. coli 16S rRNA qPCR results of patients’ peripheral blood.
After enrolment, the following items were recorded for every patient. Clinical symptoms, such as abdominal pain, abdominal distension, bowel sounds (weakened or absent), gastric retention (gastric drainage fluid >200 mL/24 h), and diarrhoea (defecation frequency >3 or 300 mL per bowel movement), were combined with other diagnostic criteria to assess the postoperative gastrointestinal dysfunction and its severity.
Before and 24 hours after the operation, 8 mL of venous blood was extracted from surgical patients. In total, 5 mL of blood was used to detect the various biomarkers of intestinal barrier dysfunction, and 3 mL of blood was obtained with anticoagulant blood containers to detect E. coli 16S DNA. Then, these blood samples were centrifuged for 5 minutes at 3,000 ×g. The supernatant was then extracted and stored at −80 °C for 30 minutes to avoid repeated freeze/thaw cycles.
Total parenteral nutrition was administered to all patients 7 days postoperatively. If the patients exhibited obvious digestive symptoms, including nausea, vomiting, diarrhoea, and distension, some drugs were used to control digestive symptoms. Otherwise, no prokinetic drugs or drugs protecting the gastrointestinal mucosa were administered.
Laboratory analysis
Human serum α-GST and DAO concentrations were measured using the ELISA Kit provided by Cusabio Biotech Co., Ltd (China). Human serum I-FABP concentrations were measured using the ELISA kit provided by R&D Systems (Minneapolis, MN). Human serum D-lactate was detected using the PicoProbe™ D-Lactate Fluorometric Assay Kit provided by BioVision Inc., Mountain View Milpitas, CA, (USA). Serum citrulline concentrations were measured by 2,4-dinitrofluorobenzene pre-column derivatization high-performance liquid chromatography (HPLC). The method is composed of the following steps: α-GST, DAO and I-FABP are detected by ELISA; D-lactic acid is detected by the colorimetric method; and citrulline is detected by HPLC. Concentrations of E. coli in peripheral blood were detected by qPCR. The amplification sequence is 16S rRNA from E. coli.
Data analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS GmbH Software, Munich, Germany). The quantitative data are expressed as the mean ± standard deviation. If the variables exhibited a non-normal distribution, the data were analysed using the rank-sum test. If the variables exhibited a normal distribution based on homogeneity variance test results, the data were analysed using a paired T-test with united variance analysis. Correlation analysis of these biomarkers and intestinal barrier dysfunction was performed using a logistic regression model, and the receiver operating characteristic (ROC) curve and area under the curve (AUC) were analysed. P<0.050 was considered statistically significant.
Results
Patients and baseline characteristics
During this study, a total of 45 patients underwent major abdominal surgery in the general surgery department. Six patients were excluded based on the following reasons: 4 patients were suffering from intestinal obstruction before the operation, and 2 patients had gastric ulcers. In total, 39 patients were enrolled. According to the diagnostic standard, 12 patients were diagnosed with intestinal barrier dysfunction, and 27 patients were diagnosed with non-intestinal barrier dysfunction. Detailed diagnostic criteria for every patient were listed in the Table S1.
Full table
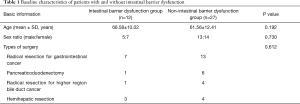
The age, sex ratio and types of surgery in the two groups are presented in Table 1. The baseline characteristics exhibited no significant differences between the intestinal barrier dysfunction group and the non-intestinal barrier dysfunction group (P>0.05).
Full table
16SRNA-qPCR results of E. coli in peripheral blood
16SRNA-qPCR results of E. coli in peripheral blood were analyzed. The 16SRNA-qPCR value of E. coli in 14 patients increased significantly (t=−2.180, P=0.048) after surgery. The positive incidence rate of E. coli translocation was 30.7% (14/39). There was no significant difference (t=1.7, P=0.102) in the 16SRNA-qPCR value of E. coli in the other 25 patients before and after surgery.
Biomarker levels in patients with suspected intestinal barrier dysfunction
Before the operation, no differences in α-GST, DAO, D-lactic acid, citrulline and I-FABP values were noted between the intestinal barrier dysfunction group and non-intestinal barrier dysfunction group.
After the operation, α-GST values were 12.48±3.89 and 11.33±4.29 ng/mL in the intestinal barrier dysfunction group and non-intestinal barrier dysfunction group, respectively (P=0.42). There was no difference in DAO values between the groups (14.10±4.77 vs. 13.65±4.33 mIU/mL, P=0.78). D-lactate values were 1.20±0.22 and 0.91±0.21 nmol/µL in the intestinal barrier dysfunction group and the non-intestinal barrier dysfunction group, respectively (P=0.001). Citrulline levels in the intestinal barrier dysfunction group were significantly lower than those in the non-intestinal barrier dysfunction group (11.25±5.41 vs. 21.56±12.15 µmol/mL, P=0.008). I-FABP levels in the intestinal barrier dysfunction group were significantly increased compared with those in the non-intestinal barrier dysfunction group (185.29±30.50 vs. 139.93±59.24 pg/mL, P=0.003).
Pearson correlation analysis was used to analyze the correlation between E. coli 16SRNA-qPCR values and biomarkers (α-GST, DAO, D-lactic acid, citrulline and I-FABP values) (Table 2). There was no significant correlation between E. coli 16S rRNA values and these biomarkers values (P>0.05).

Full table
Diagnostic accuracies of plasma biomarkers of intestinal barrier dysfunction
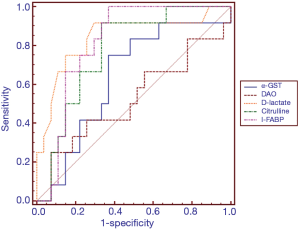
ROC analysis was performed to test the ability of the potential biomarkers to diagnose intestinal barrier dysfunction (Table 3 and Figure 1). The AUCs were increased for D-lactate and I-FABP in the diagnosis of intestinal barrier dysfunction (AUC 0.84 and 0.81). Among the other markers, citrulline exhibited the best performance (AUC 0.77). The ROC curve yielded optimal cut-off values of 0.98 nmol/µL, 143.27 pg/mL, 17.29 µmol/mL, 10.37 ng/mL and 17.90 mIU/mL for D-lactate, I-FABP, citrulline, α-GST and DAO, respectively, as biomarkers of intestinal barrier dysfunction. The sensitivity, specificity, negative predictive value, false-negative rate (FNR) and false-positive rate (FPR) were assessed using these cut-off values (Table 3). The sensitivity values of D-lactate, citrulline and I-FABP for diagnosing intestinal barrier dysfunction were high (0.91, 0.91 and 1.00, respectively). The specificity values of D-lactate and I-FABP for diagnosing intestinal barrier dysfunction were also high (0.84 and 0.81, respectively).

Full table
Screening of biomarkers for diagnosing intestinal barrier dysfunction
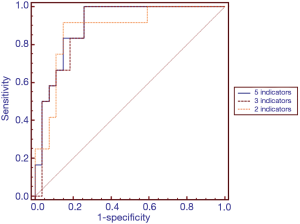
To screen effective warning biomarkers for diagnosing intestinal barrier dysfunction, multivariate logistic regression analysis was performed. The regression equation was as follows: y=−5.95−0.011x1−0.052x2+5.651x3−0.087x4+0.009x5 (x1 represents α-GST; x2 represents DAO; x3 represents D-lactate; x4 represents citrulline; and x5 represents I-FABP). ROC curve analysis was further conducted. The AUC value of the combination of five biomarkers to calculate intestinal barrier dysfunction was 0.904. The diagnostic efficacy of the five-biomarker combination was greater than that of any single biomarker.
Based on the above data, serum α-GST and DAO levels in postoperative intestinal barrier dysfunction or non-intestinal barrier dysfunction patients were not significantly different, and the diagnostic accuracy for intestinal barrier dysfunction was relatively poor, as demonstrated by ROC curve analysis results.
Thus, the potential biomarkers α-GST and DAO were excluded from further studies. The relationship between the remaining 3 biomarkers and intestinal barrier dysfunction was explored by multivariate logistic regression analysis and ROC curve analysis (Table 4 and Figure 2). The regression equation was as follows: y=−6.212+5.628x1−0.097x2+0.006x3 (x1 represents D-lactate; x2 represents citrulline; and x3 represents I-FABP). The AUC value of the three-biomarker combination to calculate intestinal barrier dysfunction was 0.892. The diagnostic accuracies of the five-biomarker combination and the three-biomarker combination were similar. The areas under the two curves were similar, and no statistically significant differences were noted (P=1.00) (Table 4 and Figure 2). The correlation between E. coli 16S rRNA qPCR values and biomarkers values shows no statistical significance (Table 5).

Full table

Full table
Discussion
This study indicates that D-lactic acid, citrulline and I-FABP were more effective biomarkers than α-GST or DAO at detecting intestinal barrier dysfunction in patients after major abdominal surgery. The combination of I-FABP, citrulline and D-lactate for identifying intestinal barrier dysfunction was more reliable than any single biomarker or other combination of biomarkers, and this combination exhibited an accuracy equivalent to that of the combination of five biomarkers in patients after major abdominal surgery.
In this study, radical resection of gastrointestinal cancer, hemihepatic resection, pancreaticoduodenectomy and radical resection for higher region bile duct cancer were included (Table 1), and intestinal barrier dysfunction was assessed after surgery. We compared the preoperative and postoperative changes in serum α-GST, DAO, D-lactate, citrulline and I-FABP levels in patients who underwent major abdominal surgery (Table 2). Compared with the concentrations of the 5 serum biomarkers preoperatively, α-GST, DAO, D-lactate and I-FABP levels were significantly increased, and citrulline levels were remarkably reduced. These results were similar to those of previous studies assessing biomarkers for the diagnosis of intestinal ischaemia (33-35). These findings suggest that monitoring changes in these biomarkers after major abdominal surgery may aid in the detection of intestinal mucosal barrier dysfunction to a certain extent.
To further evaluate the accuracy of these biomarkers in the identification of intestinal mucosal barrier dysfunction, ROC analysis was performed (Table 3 and Figure 1). In this study, the sensitivities of D-lactate, citrulline and I-FABP were greater than 0.90, but the specificities of these biomarkers were less than 0.70. The sensitivity of DAO was very low, but its specificity was remarkably high (0.92; 95% CI: 75.7–99.1). The accuracies of D-lactate and I-FABP were high, and the AUCs of both biomarkers were >0.80. Compared with previous studies assessing intestinal ischaemia, these biomarkers exhibit variable accuracies (36-41). Obviously, single biomarkers were not effective in the evaluation of intestinal mucosal barrier dysfunction given that each biomarker only partially reflects pathophysiological changes in intestinal function. However, intestinal barrier dysfunction involves a series of pathophysiological changes, including destruction of the intercellular tight junctions of intestinal mucosal epithelial cells, increased intestinal mucosal permeability, and impaired protective functions of the intestinal mucosa, causing intestinal flora and harmful metabolites to enter the blood circulation. Therefore, a combination of serum biomarkers that react to the pathophysiological changes in the intestinal mucosal barrier may improve the diagnostic performance of the assessment of intestinal mucosal barrier dysfunction.
In further statistical analyses, a binary logistic regression model and ROC curve analyses were used to explore the diagnostic performance of different combinations of five serum biomarkers in the assessment of intestinal barrier dysfunction. We found that a combination of 3 biomarkers (I-FABP, citrulline and D-lactate) and a combination of 5 biomarkers (α-GST, DAO, I-FABP, citrulline and D-lactate) exhibited the best diagnostic performance among different biomarker combinations. There was no significant difference in diagnostic accuracy between the two combinations in the diagnosis of intestinal barrier dysfunction (P=1.00). According to the principle of clinical practicality, the combination of 3 biomarkers (I-FABP, citrulline and D-lactate) is the first choice for the detection of intestinal barrier dysfunction.
This study has several methodological differences from previous studies. (I) It is the first study to screen and combine serum biomarkers to improve the diagnostic performance in the assessment of intestinal barrier dysfunction. In many previous studies in patients with suspected intestinal ischaemia (36-38,42), researchers paid more attention to the diagnostic performance of a single biomarker. Even if some researchers assessed numerous biomarkers in some studies (39-41), they did not combine different biomarkers to improve the diagnostic performance. (II) The diagnostic standard of intestinal barrier dysfunction was reported clearly in this study. Only three clinical studies evaluating the diagnostic performance of serum biomarkers in the detection of intestinal barrier dysfunction have been previously reported, and the authors of one study regrettably did not describe the diagnostic standard of intestinal barrier dysfunction (23). Acute gastrointestinal injury (AGI) grade was used in another study as the diagnostic standard of intestinal barrier dysfunction (24). (III) After surgery, we chose to provide parenteral nutrition instead of enteral nutrition or oral feeding. The latter feeding modality can protect the intestinal mucosal barrier and disturb the biomarker testing results.
Some limitations of our study should be noted. First, we screened only 5 biomarkers that exhibited relatively high accuracies in the detection of intestinal barrier dysfunction and were supported by published evidence. Other biomarkers, including LPS, CRP, and D-Dimer, had a relatively lower accuracy reported in published papers and therefore were not considered in our study due to funding limitations. Thus, it is possible that we missed some effective biomarkers. Second, no uniform standard is currently available for the clinical diagnosis of intestinal barrier dysfunction. We referenced the diagnostic standard of intestinal barrier dysfunction recommended by the experts from the Digestive Disease Branch of Chinese Medical Association (26) and a study performed in China (26). However, the diagnostic standard is not suitable to assess patients from other countries. Third, we used E. coli 16S DNA-qPCR to evaluate intestinal bacterial translocation, which is important in the detection of intestinal mucosal barrier dysfunction and was supported by numerous researchers in many studies (30,31). The E. coli 16S rRNA qPCR results may not completely represent bacterial translocation from other bacteria in the intestinal lumen. Fourth, as we performed this study in patients undergoing major abdominal surgery, we are not sure whether these biomarkers are suitable for detecting intestinal barrier dysfunction in other diseases. Further well-designed studies in patients with severe burns, trauma, infection, and shock are needed to confirm these findings. Fifth, even if the sample size of this exploratory study is sufficient to identify the differences in intestinal barrier dysfunction identification, the sample size was small. To promote the use of these biomarkers in the detection of intestinal mucosal barrier dysfunction in the clinic, a large sample size in a multicentre prospective clinical study is required to support the findings in this study. Although this study has some limitations, it provides a new approach to the clinical diagnosis of intestinal mucosal barrier dysfunction.
In this exploratory study, we found that the combination of D-lactate, citrulline and I-FABP was a more effective indicator to identify intestinal mucosal barrier dysfunction in patients undergoing major abdominal surgery. This technique should be recommended to help doctors identify patients with intestinal mucosal barrier dysfunction in the clinic.
Acknowledgments
Funding: This study was supported by the Foundation of Research Center for Surgical Clinical Nutrition in Yun-Nan Province, Professor Yang Hua Research Station in Yun-Nan Province (no. 2015IC034), and The National Natural Science Fund (no. 81160114, 81860098).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University. All enrolled subjects read information on and understood the purpose of this study and then provided informed consent.
References
- Barreau F, Hugot JP. Intestinal barrier dysfunction triggered by invasive bacteria. Curr Opin Microbiol 2014;17:91-8. [Crossref] [PubMed]
- Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [Crossref] [PubMed]
- Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med 2014;20:214-23. [Crossref] [PubMed]
- Klingensmith NJ, Coopersmith CM. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit Care Clin 2016;32:203-12. [Crossref] [PubMed]
- Kaplowitz N. Physiological significance of glutathione S-transferases. Am J Physiol 1980;239:G439-44. [PubMed]
- van Haren F M, Sleigh J W, Pickkers P, et al. Gastrointestinal perfusion in septic shock. Anaesth Intensive Care 2007;35:679-94. [Crossref] [PubMed]
- Sun XQ, Fu X B, Zhang R, et al. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World J Gastroenterol 2001;7:555-8. [Crossref] [PubMed]
- Pan L, Wang X, Li W, et al. The intestinal fatty acid binding protein diagnosing gut dysfunction in acute pancreatitis: a pilot study. Pancreas 2010;39:633-8. [Crossref] [PubMed]
- Crenn P, Hanachi M, Neveux N, et al. Circulating citrulline levels: a biomarker for intestinal functionality assessment. Ann Biol Clin (Paris) 2011;69:513-21. [Crossref] [PubMed]
- Crenn P, Coudraylucas C, Thuillier F, et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000;119:1496-505. [Crossref] [PubMed]
- Papadia C, Sherwood RA, Kalantzis C, et al. Plasma citrulline concentration: a reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol 2007;102:1474-82. [Crossref] [PubMed]
- Wedlake L, Mcgough C, Hackett C, et al. Can biological markers act as non-invasive, sensitive indicators of radiation-induced effects in the gastrointestinal mucosa? Aliment Pharmacol Ther 2008;27:980-7. [Crossref] [PubMed]
- Ruiz P, Tryphonopoulos P, Island E, et al. Citrulline Evaluation in Bowel Transplantation. Transplant Proc 2010;42:54-6. [Crossref] [PubMed]
- Schellekens DH, Grootjans J, Dello SA, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol 2014;48:253-60. [Crossref] [PubMed]
- Bingold T M, Franck K, Holzer K, et al. Intestinal Fatty Acid Binding Protein: A Sensitive Marker in Abdominal Surgery and Abdominal Infection. Surg Infect (Larchmt) 2015;16:247-53. [Crossref] [PubMed]
- Hyšpler R, Tichá A, Kaška M, et al. Markers of Perioperative Bowel Complications in Colorectal Surgery Patients. Dis Markers 2015;2015:428535. [Crossref] [PubMed]
- Ng EW, Poon TC, Lam HS, et al. Gut-associated biomarkers L-FABP, I-FABP, and TFF3 and LIT score for diagnosis of surgical necrotizing enterocolitis in preterm infants. Ann Surg 2013;258:1111-8. [Crossref] [PubMed]
- Grimaldi D, Guivarch E, Neveux N, et al. Markers of intestinal injury are associated with endotoxemia in successfully resuscitated patients. Resuscitation 2013;84:60. [Crossref] [PubMed]
- van der Voort PH, Westra B, Wester JP, et al. Can serum L-lactate, D-lactate, creatine kinase and I-FABP be used as diagnostic markers in critically ill patients suspected for bowel ischemia. BMC Anesthesiol 2014;14:111. [Crossref] [PubMed]
- Fukudome I, Kobayashi M, Dabanaka K, et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol 2014;47:100-7. [Crossref] [PubMed]
- Karabulut KU, Narci H, Gul M, et al. Diamine oxidase in diagnosis of acute mesenteric ischemia. Am J Emerg Med 2013;31:309-12. [Crossref] [PubMed]
- Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care 2016;22:152-60. [PubMed]
- Barbeiro HV, Machado M, De HS, et al. Reduction of venous pressure during the resection of liver metastases compromises enteric blood flow: IGFBP-1 as a novel biomarker of intestinal barrier injury. Clinics (Sao Paulo) 2017;72:645. [Crossref] [PubMed]
- Li H, Ying C, Huo F, et al. Association between acute gastrointestinal injury and biomarkers of intestinal barrier function in critically ill patients. BMC Gastroenterol 2017;17:45. [Crossref] [PubMed]
- Lau E, Marques C, Pestana D, et al. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr Metab (Lond) 2016;13:31. [Crossref] [PubMed]
- Guo YY, Liu ML, He XD, et al. Functional changes of intestinal mucosal barrier in surgically critical patients. World J Emerg Med 2010;1:205-8. [PubMed]
- Sharma A, Chatterjee S, Wu WC, et al. Risk of cardiac mortality and myocardial infarctions in bmi <20 kg/m2: can rates of myocardial infarctions explain the higher cardiac mortality? Am Coll Cardiol 2013;61:E1533. [Crossref]
- Wu Q, Xiao Z, Cheng Z, et al. Effects of Roux-en-Y gastric bypass on blood glucose and gastrointestinal hormones in Chinese type 2 diabetic patients with BMI ≥28 kg/m2. C Proceedings of the 16th National Academic Conference of Diabetes Branch of Chinese Medical Association. 2012.
- Digestive Disease Branch of Chinese Medical Association. Suggestion on management of intestinal barrier dysfunction. Chinese Journal of Digestion 2006;26:620.
- O’Boyle CJ, Macfie J, Mitchell CJ, et al. Microbiology of bacterial translocation in humans. Gut 1998;42:29. [Crossref] [PubMed]
- Wu LM, Sankaran SJ, Plank LD, et al. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg 2014;101:1644-56. [Crossref] [PubMed]
- Qiao Z, Li Z, Li J, et al. Bacterial Translocation and Change in Intestinal Permeability in Patients after Abdominal Surgery. J Huazhong Univ Sci Technolog Med Sci 2009;29:486-91. [Crossref] [PubMed]
- Rosero O. Acute mesenteric ischemia: Do biomarkers contribute to diagnosis? Orv Hetil 2014;155:1615-23. [Crossref] [PubMed]
- Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis 2012;33:355-61. [Crossref] [PubMed]
- Powell A, Armstrong P. Plasma biomarkers for early diagnosis of acute intestinal ischemia. Semin Vasc Surg 2014;27:170-5. [Crossref] [PubMed]
- Khadaroo RG, Fortis S, Salim SY, et al. I-FABP as Biomarker for the Early Diagnosis of Acute Mesenteric Ischemia and Resultant Lung Injury. PLoS One 2014;9:e115242. [Crossref] [PubMed]
- Kanda T, Tsukahara A, Ueki K, et al. Diagnosis of ischemic small bowel disease by measurement of serum intestinal fatty acid-binding protein in patients with acute abdomen: a multicenter, observer-blinded validation study. J Gastroenterol 2011;46:492-500. [Crossref] [PubMed]
- Acosta S, Nilsson TK, Björck M. D‐dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br J Surg 2004;91:991-4. [Crossref] [PubMed]
- Goswami P, Sonika U, Moka P, et al. Intestinal Fatty Acid Binding Protein and Citrulline as Markers of Gut Injury and Prognosis in Patients With Acute Pancreatitis. Pancreas 2017;46:1275-80. [Crossref] [PubMed]
- Matsumoto S, Sekine K, Funaoka H, et al. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg 2014;101:232. [Crossref] [PubMed]
- Shi H, Wu B, Wan J, et al. The role of serum intestinal fatty acid binding protein levels and D-lactate levels in the diagnosis of acute intestinal ischemia. Clin Res Hepatol Gastroenterol 2015;39:373-8. [Crossref] [PubMed]
- Poeze M, Froon AH, Greve JW, et al. D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg 1998;85:1221-4. [Crossref] [PubMed]