
Clinical epidemiology and outcomes of biliary tract infections caused by Klebsiella pneumoniae
Introduction
Biliary tract infection (BTI), including cholangitis and cholecystitis, is a common cause of bacteremia, especially for patients with underlying structural abnormalities (1). BTI is associated with high morbidity and mortality especially when diagnosis or treatment is delayed (2-4). Acute BTI requires a combination of medical and surgical therapy, including adequate antimicrobial therapy and biliary decompression. Mortality for patients with BTIs and bacteremia has been reported to be 10–20% (5-7), despite newer antibiotics and biliary decompression methods.
The pathogens in BTIs are frequently polymicrobial, but the most common organisms are Enterobacteriaceae (i.e., Escherichia coli and Klebsiella spp.) ascending from the gut flora (8,9). Klebsiella pneumoniae (KP) is known to asymptomatically colonize the skin, and respiratory and gastrointestinal tracts and is recognized to have a wide range of clinical manifestations including urinary tract infections, pneumonia, soft tissue infections, bacteremia, abscesses, and intra-abdominal infections. Additionally, in recent decades, the numbers of antibiotic-resistant pathogens have increased steadily. Winokur et al. (10) reported that 8% (in the US) to 45% (in Latin America) of KP isolates and 3.3% (in the US) to 8.8% (in Latin America) of E. coli isolates were extended-spectrum beta-lactamase (ESBL) phenotypes. Furthermore, the emergence of multidrug-resistant KP has become a great concern worldwide and has resulted in a dramatic increase in research into reservoirs and risk factors for KP infection (11,12).
Several previous studies have evaluated bacteremia in BTI; however, information regarding BTIs caused by KP, especially those confirmed with bile culture, susceptibility testing, bacterial species identification, or specific anti-infection strategies are rare. Therefore, improving BTI treatments requires further research. Furthermore, the impact of ESBL-producing KP on patients’ clinical outcomes has also not been well described in patients with BTIs.
Because inadequate antimicrobial therapy is associated with an increased risk of overall mortality, it is necessary to determine the pathogen and its drug-susceptibility early in the disease process. For this purpose, we performed a single-center retrospective study to evaluate the clinical epidemiology of patients with BTIs caused by KP. We described patients’ demographics, susceptibility profiles, 30-day mortality, and the prognostic factors related to survival in these patients. In addition, we investigated the different clinical outcomes in patients with malignant vs. nonmalignant biliary obstructions.
Methods
Study setting
This retrospective study was performed at a tertiary care university teaching hospital, Ren Ji Hospital, affiliated with the Shanghai Jiaotong University of Medicine, in Shanghai, China. The study period was from May 1, 2012 to June 30, 2017. We reviewed patients’ medical and microbiological records to identify consecutive patients with BTIs. All patients were ≥16 years old and had positive bile culture results. Infections were confirmed by two clinical microbiologists and managed in conjunction with their medical or surgical teams. The specimens for bile culture were obtained surgically in 66/119 (55.5%) patients, by endoscopic retrograde cholangiopancreatography in 13/119 (10.9%) patients, and by percutaneous transhepatic biliary drainage in 40/119 (33.6%) patients. The bile specimen sampling method was chosen by the attending gastroenterologist, not by the investigators. Patients with recurrent infections and other sources of infection were excluded. Clinical manifestations were determined from patients’ medical charts.
Definition
Cholecystitis was diagnosed based on the presence of fever, right upper quadrant pain, and imaging findings, such as those of ultrasonography or computed tomography. Cholangitis was diagnosed based on the following: (I) the presence of fever with upper quadrant pain; (II) radiological (computed tomographic or sonographic) or endoscopic evidence of biliary tract obstruction secondary to a stone or a benign or malignant stricture; and (III) laboratory findings of hyperbilirubinemia and an elevated serum alkaline phosphatase level.
Data describing patients’ clinical variables were collected from their computerized medical records and included age, sex, underlying medical conditions (malignancy, hypoproteinemia, chronic liver disease, diabetes mellitus, solid organ transplantation), and laboratory findings (C-reactive protein, procalcitonin, erythrocyte sedimentation rate, direct and total bilirubin direct, alanine aminotransferase). Patients with BTI (benign/malignant) were admitted to our institution with a positive culture for an ESBL-producing or nonproducing isolate of KP (ESBL-positive and ESBL-negative, respectively).
Initial empirical antimicrobial therapy was defined as treatment with at least one agent to which the isolate was susceptible in vitro according to the Clinical and Laboratory Standards Institute breakpoints (13).
Septic shock was defined as sepsis associated with systemic inflammatory response syndrome (any two of the following: tachypnea >20 breaths/min, white blood cell count <4,000 or >1,200 cells/µL, heart rate >90 beats/min, and fever >38.0 °C or hypothermia <36.0 °C), organ dysfunction (renal failure, liver dysfunction, changes in mental status, and elevated serum lactate) and persistent hypotension after volume replacement (14).
Immunosuppression was defined as leukopenia (granulocytes <0.5×109/L), and otherwise impaired immunity (e.g., chronic corticosteroid therapy, chemotherapy and underlying malignancy, dialysis or systemic disease such as leukemia, solid organ transplantation, or acquired immunodeficiency in patients with granulocytes >0.5×109/L).
Mortality was defined as death from any cause within 30 days from the onset of symptoms. Chronic liver disease was defined as liver disease of >6 months’ duration, namely, chronic hepatitis, autoimmune liver disease, or cirrhosis.
Microbiology
KP isolates were confirmed using the Vitek 2 Advanced Expert System (bioMèrieux, Marcy l’Etoile, France), and antibiotic susceptibility was performed using the agar disc diffusion method (Kirby-Bauer). Antibiotic susceptibility was interpreted according to the European Committee on Antimicrobial Susceptibility Testing guidelines (15).
Data analysis
Continuous variables were compared using the Mann-Whitney U test, and the chi-square test or Fisher’s exact test was used to compare categorical variables. All significant variables with P<0.10 in the univariate analysis were considered candidates for entry into a forward stepwise multivariate logistic regression model; forward stepwise selection was performed to develop the final model. A P value less than 0.05 was considered statistically significant. All data were analyzed using the IBM SPSS Statistics for Windows (version 19.0; IBM Corp., Armonk, NY, USA). ORs and 95% CIs were calculated to evaluate the strength of any association.
Results
A total of 119 patients had confirmed KP isolates during the study period. Among these patients, the median age was 65.15 years (range, 16–94 years) and 60 (50.4%) were men.
The demographic and clinical characteristics of both survivors and nonsurvivors with KP-related BTIs are shown in Table 1. Of the 119 patients, 112 (94.1%) survived and 7 (5.9%) died within 30 days of onset. When we compared the comorbid conditions in the two groups using univariate analysis, nonsurvivors were significantly more likely to be older (66.46±22.34 vs. 46±14.84 years; P=0.001), and have hypoproteinemia (5/7, 71.4% vs. 21/112, 18.8%; P=0.006), immunosuppression (3/7, 42.9% vs. 4/112, 3.6%; P=0.004), solid tumors (5/7, 71.4% vs. 20/112, 17.9%; P=0.004), bloodstream infections (6/7, 85.7% vs. 22/112, 19.6%; P=0.001) and lower surgery rates (1/7, 14.3% vs. 66/112, 58.9%; P=0.042) compared with survivors, respectively. However, we found no significant independent risk factor for mortality in the multivariate analysis.
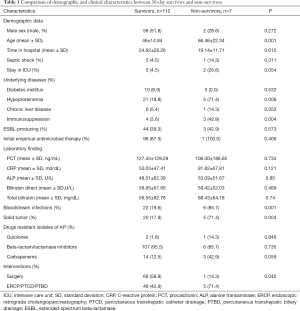
Full table
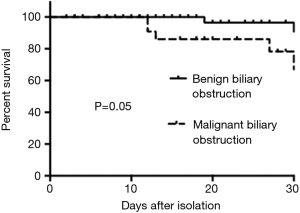
The demographic and clinical features of the patients with malignant biliary obstruction are summarized in Table 2. Univariate analysis revealed that the patients with malignant biliary obstruction were significantly more likely to have chronic liver disease (P=0.035) compared with controls, and the mortality rate was higher in patients with malignant biliary obstruction (P=0.05) (Figure 1). Comparing patients’ laboratory findings, the malignant biliary group had higher alkaline phosphatase, and direct and total bilirubin levels. Multivariate logistic regression analysis showed that chronic liver disease was an independent risk factor in patients with malignant biliary disease (OR, 2.431; 95% CI, 1.834–4.031; P=0.001).
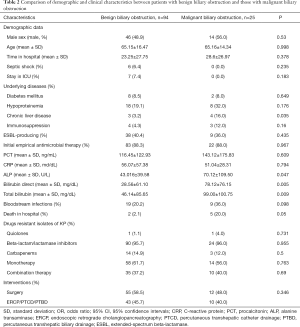
Full table
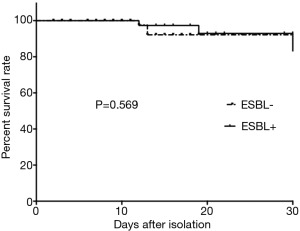
Forty-seven KP strains (39.5%) were ESBL-positive (Table 3), and the ESBL-positive group had a higher rate of stay in ICU (P=0.015). The ESBL-positive group also had a significantly longer hospital stay than ESBL-negative patients (P=0.037). The initial empirical antimicrobial drugs and the number of drug-resistant KP isolates were not statistically significantly different between the ESBL-positive and ESBL-negative groups. The ESBL-positive group had a higher 30-day mortality rate than the ESBL-negative group, but no association was found between ESBL-positive isolates and 30-day mortality. The rate of antibiotic resistance to beta-lactam/lactamase inhibitors and carbapenems for ESBL-producing KP was 95.7% and 21.3%, respectively, but there was no statistically significant difference between the two groups (Figure 2).
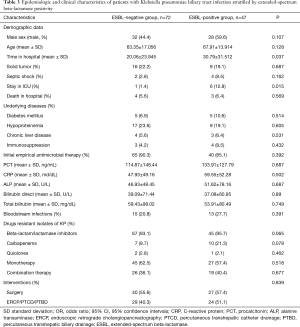
Full table
Discussion
The most common infecting organisms in BTIs are E. coli and KP (1), but few studies have focused specifically on the clinical epidemiology and outcomes of BTIs caused by KP. Kim et al. (16) reported in 2013 that the prevalence of the ESBL-positive phenotype in KP and E. coli isolated from blood and/or bile cultures was 13.2% (21/159 isolates), and that the prevalence of ESBL-producing KP was 25.3% (19/75 isolates). Increased bacterial antibiotic exposure from antibiotic overuse, misuse, or even appropriate use, has led to increased antibiotic resistance (17). Sung et al. (18) found that the prevalence of ESBL-producing E. coli and KP strains isolated from patients’ blood cultures increased markedly from 2.3% (2/86) in 2000–2004 to 43.9% (58/132) in 2005–2009. In the present study, we found 39.5% (47/119 isolates) of KP isolated from bile cultures in patients with BTIs were ESBL-producing strains, while the percentage of ESBL-producing KP was 10.7% (3/28 isolates) in another study from China (19). ESBL-producing KP is widespread throughout the world, but the prevalence of clinical isolates varies from area to area; clonal spread may be the key cause (20).
Few studies have discussed 30-day mortality in patients with ESBL-positive KP. Kim et al. reported that 30-day mortality was significantly higher for ESBL-positive strains (3/21, 14.3%) compared with ESBL-negative strains (4/138, 2.9%) (16). Additionally, Tumbarello et al. (21) reported that a delay in beginning effective antibiotic therapy was a significant predictor of 21-day mortality in patients with bloodstream infections caused by ESBL-producing Enterobacteriaceae. Mezler and Peterson (22) reported that the length of hospital stay between patients with ESBL-producing and nonESBL-producing E. coli was not significantly different. In our study, the ESBL-positive group had a higher rate of stay in ICU and a significantly longer hospital stay as well as a 30-day mortality of 6.4% (3/47), which was lower than in the cited studies. The differences in 30-day mortality and other clinical outcomes between our study and previous studies may have several explanations. First, patients’ characteristics differed between studies. In our study, patients had BTIs caused by KP, while other studies focused on adult patients with Enterobacteriaceae (E. coli and KP). Second, the study by Mezler and Peterson (22) evaluated only E. coli bacteremia, and Kim et al.’s study included patients with blood (88/159, 55.3%) and/or bile (71/159, 44.7%) cultures positive for KP and E. coli. However, the proportions of patients with bacteremia in our study were 23.5% (28/119) and 27.7% (13/47) in the ESBL-positive group and ESBL-negative group, respectively (16). Third, over half of patients with ESBL-producing Enterobacteriaceae were not provided with adequate antibiotic therapy (11/21, 52.4%) within 72 hours in Kim et al.’s study (16); in our study, this proportion was 85.1% (40/47).
In our study, nonsurvivors were significantly more likely to be older, and have hypoproteinemia, solid organ transplantation, solid tumors, bloodstream infections, and surgery than were survivors. However, we found no significant difference in antibiotic resistance between the two groups. Some investigators have concluded that antibiotic resistance is a significant independent predictor of mortality in bacteremia (23,24). However, mortality related to ESBL-producing KP bacteremia was similar to that of nonESBL-producing KP bacteremia in other studies (25,26). Notably, mortality for patients with biliary infection was significantly lower than for other patients in one study; none died from ESBL-KP bacteremia with BTI (25). Two previous studies also showed that antibiotic resistance was not significantly associated with overall survival in patients with BTIs (16,18). These contrary findings likely reflect that a nonantibiotic approach, such as with effective biliary decompression and general supportive care, is also important during BTI treatment.
Malignant biliary obstruction is one of the most common complications of biliary malignant tumors (27), and malignant biliary obstruction with biliary infection can lead to poor clinical outcomes (28,29). Sung et al.’s study showed that 95.9% (47/49) of the study’s patients experienced malignant biliary obstruction, and the presence of a solid tumor was considered the most powerful predictor of mortality (adjusted OR: 9.82) (18). This is consistent with our study, in which we found much higher mortality (5/25, 20%) in patients with malignant biliary obstruction than that in the benign biliary obstruction group (2/94, 2.1%). Although there was a significant difference in overall survival between patients with benign biliary obstruction vs. malignant biliary obstruction, malignant biliary obstruction was not an independent risk factor for mortality by multivariate analysis. The potential causes for this difference may be that some severe patients were treated with biliary decompression, and that the duration of observation in this study was limited to hospitalization.
This study has several potential limitations. First, this was a retrospective study with limited patient numbers, and was performed in a single center. Second, patients with BTIs in this study were mainly determined by bile culture, while only a small number of patients were complicated with bloodstream infection. Therefore, the mortality rate of BTIs caused by KP may be underestimated. Finally, we did not distinguish patients as having community-acquired vs. nosocomial BTIs, and the impact of previous antibiotic use on emergence of antibiotic resistance is unknown.
Conclusions
We performed an observational study of KP-related BTIs based on bile culture. Patients with BTIs caused by KP had a high prevalence of the ESBL-positive KP phenotype, while antibiotic resistance was not significantly associated with overall survival. Patients with malignant biliary obstruction had higher mortality, and chronic liver disease was an independent risk factor.
Acknowledgments
We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Renji Hospital (Shanghai Jiao Tong University School of Medicine), and the study met the guidelines of the Declaration of Helsinki.
References
- Melzer M, Toner R, Lacey S, et al. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad Med J 2007;83:773-6. [Crossref] [PubMed]
- Bornman PC, van Beljon JI, Krige JEJ. Management of cholangitis. J Hepatobiliary Pancreat Surg 2003;10:406-14. [Crossref] [PubMed]
- Anon. . What if it’s acute cholangitis? Drugs Therapy Bulletin 2005;43:8.
- Lee CC, Chang IJ, Lai YC, et al. Epidemiology and prognostic determinants of patients with bacteraemic cholecystitis or cholangitis. Am J Gastroenterol 2007;102:563-9. [Crossref] [PubMed]
- Wada K, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007;14:52-8. [Crossref] [PubMed]
- Lai EC, Tam PC, Paterson IA, et al. Emergency surgery for severe acute cholangitis. The high-risk patients. Ann Surg 1990;211:55-9. [Crossref] [PubMed]
- Podnos YD, Jimenez JC, Wilson SE. Intra-abdominal sepsis in elderly persons. Clin Infect Dis 2002;35:62-8. [Crossref] [PubMed]
- Claesson BE, Holmlud DE, Matzsch TW. Microflora of the gallbladder related to duration of acute cholecystitis. Surg Gynecol Obstet 1986;162:531-535. [PubMed]
- Shimada K, Noro T, Inamatsu T, et al. Bacteriology of acute obstructive suppurative cholangitis of the aged. J Clin Microbiol 1981;14:522-6. [PubMed]
- Winokur PL, Canton R, Casellas JM, et al. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis 2001;32:S94-103. [Crossref] [PubMed]
- Centres for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013: Centres for Disease Control and Prevention, US Department of Health and Human Services, 2013.
- Wiener-Well Y, Rudensky B, Yinnon AM, et al. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect 2010;74:344-9. [Crossref] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document. Wayne, PA: Clinical and Laboratory Standards Institute, 2014:M100-S24.
- Russell JA. Management of sepsis. N Engl J Med 2006;355:1699-713. [Crossref] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2016) Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0.Home page. Available online: http://www.eucast.org
- Kim HJ, Park JH, Park DI, et al. Clinical Impact of Extended-Spectrum b-Lactamase-Producing Enterobacteriaceae in Patients with Biliary Tract Infection. Dig Dis Sci 2013;58:841-9. [Crossref] [PubMed]
- Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008;46:155-64. [Crossref] [PubMed]
- Sung YK, Lee JK, Lee KH, et al. The Clinical Epidemiology and Outcomes of Bacteremic Biliary Tract Infections Caused by Antimicrobial-Resistant Pathogens. Am J Gastroenterol 2012;107:473-83. [Crossref] [PubMed]
- Yu H, Gun ZG, Xing WG, et al. Bile Culture and Susceptibility Testing of Malignant Biliary Obstruction via PTBD. Cardiovasc Intervent Radiol 2012;35:1136-44. [Crossref] [PubMed]
- Rodríguez-Baño J, Pascual A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther 2008;6:671-83. [Crossref] [PubMed]
- Tumbarello M, Sanguinetti M, Montuori E, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteria-ceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007;51:1987-94. [Crossref] [PubMed]
- Melzer M, Peterson I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect 2007;55:254-9. [Crossref] [PubMed]
- Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin Infect Dis 2004;39:812-8. [Crossref] [PubMed]
- Kim YK, Pai H, Lee HJ, et al. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother 2002;46:1481-91. [Crossref] [PubMed]
- Kim BN, Woo JH, Kim MN, et al. Clinical implications of extended- spectrum beta-lactamase-producing Klebsiella pneumoniae bacteraemia. J Hosp Infect 2002;52:99-106. [Crossref] [PubMed]
- Menashe G, Borer A, Yagupsky P, et al. Clinical significance and impact on mortality of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolates in nosocomial bacteremia. Scand J Infect Dis 2001;33:188-93. [Crossref] [PubMed]
- Brown KT, Covey AM. Management of malignant biliary obstruction. Tech Vasc Interv Radiol 2008;11:43-50. [Crossref] [PubMed]
- Wang Q, Gurusamy KS, Lin H, et al. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2008.CD005444. [PubMed]
- Westwood DA, Fernando C, Connor SJ. Internal-external percutaneous transhepatic biliary drainage for malignant biliary obstruction: a retrospective analysis. J Med Imaging Radiat Oncol 2010;54:108-10. [Crossref] [PubMed]


