Perioperative sedative use is not associated with acute kidney injury after total hip or knee arthroplasty
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are usually performed to treat advanced arthritis, with their prevalence increasing steadily (1). Thus, early recovery after surgery (ERAS) of patients who underwent THA or TKA has become an important clinical issue (2). From the ERAS perspective, occurrence of postoperative complications must be reduced in patients who underwent THA or TKA. An important complication is acute kidney injury (AKI), with incidence rates after THA or TKA ranging from 1.85% to 6.85% (3-5). Considering that AKI occurrence after TKA is associated with worse long-term outcomes, including higher readmission rate and mortality (6), AKI prevention after THA or TKA is clinically very important.
Although TKA or THA was generally previously performed under general anesthesia, regional anesthesia has become a recent global trend for THA or TKA (7). In regional anesthesia, anesthesiologists perform sedation to reduce patient anxiety (8). Sedative agents commonly used for such cases include midazolam, propofol, and dexmedetomidine (8). Of these, propofol has been reported to have anti-inflammatory and protective effects against renal ischemia, while reducing AKI incidence in critically ill patients or patients who underwent cardiac surgery (9,10). Moreover, a recent meta-analysis reported that perioperative administration of dexmedetomidine reduces AKI in adult cardiac surgery patients (11), and the mechanism is presumed to involve dexmedetomidine’s anti-inflammatory and protective effects against renal ischemia (12,13).
Thus, the use of propofol or dexmedetomidine may decrease postoperative AKI incidence with increasing dosage. However, no study has been done to investigate this. Thus, this study aimed to investigate the associations between dosage of propofol and dexmedetomidine used during TKA or THA under spinal synesthesia and postoperative AKI incidence. We hypothesized that as propofol or dexmedetomidine dosage increases, the incidence of postoperative AKI will decrease.
Methods
This retrospective study was conducted after approval by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (SNUBH) (Approval Number: B-1711/432-103). The requirement to obtain informed consent was waived by the IRB because of the retrospective nature of the study. The STROBE guidelines for reporting on observational cohort studies were followed. Medical records of adult patients aged 18 years or older who underwent TKA or unilateral THA under spinal anesthesia at SNUBH between January 1, 2007, and June 30, 2016, were reviewed in the study. Patients whose serum creatinine level was not measured within 1 month prior to the procedure or in postoperative days (POD) 0–3 were excluded from the analysis.
Anesthesia for TKA or THA
At SNUBH, hyperbaric local anesthetics have been used with 10–15 µg of fentanyl for unilateral TKA or THA procedure. The aimed block level was T10. After attaining adequate block level, midazolam, propofol, or dexmedetomidine was used to sedate patients during the procedure. The use and dosage of each sedative was at the discretion of the anesthesiologist.
Diagnosis of AKI in POD 0–3
We followed the criteria and grading of Kidney Disease: Improving Global Outcomes for the diagnosis of AKI (14). However, taking into consideration the varying duration of urinary catheterization, urine output was excluded, and only serum creatinine was used for the AKI diagnosis. Serum creatinine measured preoperatively within 1 month before the procedure was used as the baseline value, while serum creatinine measured in POD 0–3 was used as postoperative serum creatinine.
Stage 1 AKI was diagnosed when there was ≥0.3 mg/dL increase in serum creatinine or when serum creatinine increase to ≥1.5–1.9. Stage 2 AKI was defined as ≥2.0–2.9-fold increase in serum creatinine from preoperative serum creatinine, and stage 3 AKI was defined as ≥3-fold increase in serum creatinine from preoperative serum creatinine, ≥4.0 mg/dL increase, or new initiation of renal replacement therapy (RRT) within 48 h.
Other measurements and outcome
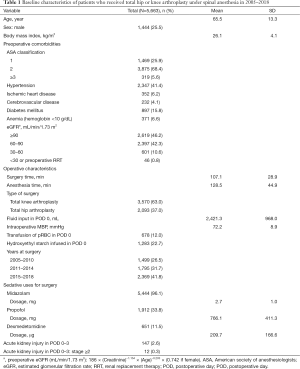
Baseline characteristics [age (year), sex, and body mass index (kg/m2)], preoperative comorbidities [American Society of Anesthesiologists (ASA) physical status, hypertension, ischemic heart disease, cerebrovascular disease, diabetes mellitus, anemia, estimated glomerular filtration rate (eGFR, mL/min/1.73 m2)], operative characteristics [surgery and anesthesia time 16, type of surgery (TKA or THA), total fluid input in POD 0 (15), intraoperative mean blood pressure (mmHg), transfusion of packed red blood cell (pRBC) in POD 0, years at surgery] of the patients were collected. The Modification of Diet in Renal Disease formula was used for the preoperative eGFR calculation (16): Preoperative eGFR (mL/min/1.73 m2): 186× (preoperative creatinine)−1.154 × (age)−0.203 × (0.742 if female).
Statistical analysis
Baseline characteristics of patients were presented as mean with standard deviation (SD) or number with percentage. First, we performed univariable logistic regression analysis to examine individual associations between AKI in POD 0–3 and each variable. Variables with P<0.2 from this univariable logistic regression analysis were selected and included in the final multivariable logistic regression analysis, in addition to our key variables of interest, such as sedative use (midazolam, propofol, and dexmedetomidine). Multi-collinearity was not detected in this multivariable logistic regression model (all variance inflation factors <0.2), and the goodness of fit of the model was tested using Hosmer and Lemeshow statistics. All statistical analysis was performed using IBM SPSS version 24.0 and P<0.05 was considered statistically significant.
Results
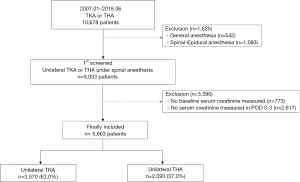
Among 10,678 patients who underwent TKA or THA between January 2007 and June 2018, 542 were given general anesthesia and 1,083 were given spinal-epidural anesthesia, and hence excluded. Of the remaining 9,053 patients, 773 were excluded because they did not have baseline serum creatinine recorded during the preoperative period, and 2,617 were excluded because they did not have serum creatinine recorded in POD 0–3. The final sample for analysis included 5,663 patients, of which 3,570 underwent TKA (63.0%) and 2,093 underwent THA (37.0%) (Figure 1). The baseline characteristics of these patients are presented in Table 1. In POD 0–3, 147 patients (2.6%) developed total AKI, and of these patients, 12 (0.3%) had AKI stage ≥2. For sedation, midazolam was used in 5,444 patients (96.1%) with mean (SD) dosage of 2.7 mg (1.0), propofol was used in 1,912 patients (33.8%) with mean (SD) dosage of 766.1 mg (411.3), and dexmedetomidine was used in 651 patients (11.5%) with mean (SD) dosage of 209.7μg (166.6).
Full table
Perioperative sedative use and AKI in POD 0–3
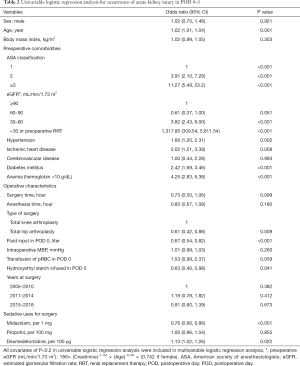
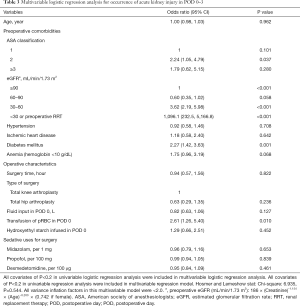
The results of the univariable logistic regression analysis for AKI in POD 0–3 are presented in Table 2. Midazolam dosage (OR: 0.76, 95% CI: 0.66, 0.88; P<0.001) and dexmedetomidine dosage (OR: 1.13, 95% CI: 1.02, 1.26; P=0.022) were associated with occurrence of AKI, while propofol dosage (OR: 1.00, 95% CI: 0.96, 1.04; P=0.855) was not associated with occurrence of AKI. The results of the multivariable logistic regression analysis, which include variables with P<0.2 from the univariable logistic regression model, are presented in Table 3. The multivariable logistic regression analysis showed that midazolam dosage (OR: 0.96, 95% CI: 0.79, 1.16; P=0.653), propofol dosage (OR: 0.99, 95% CI: 0.94, 1.05; P=0.839), and dexmedetomidine dosage (OR: 0.95, 95% CI: 0.84, 1.09; P=0.461) were not significantly associated with AKI occurrence in POD 0–3.
Full table
Full table
In the multivariable logistic regression analysis, factors significantly associated with AKI were ASA classification 2 (vs. 1) (OR: 2.24, 95% CI: 1.05, 4.79; P=0.037); preoperative eGFR, 30–60 (vs. ≥90) (OR: 3.62, 95% CI: 2.19, 5.98; P<0.001), <30, or preoperative RRT (vs. ≥90) (OR: 1,096.1, 95% CI: 232.5, 5,166.8; P<0.001); diabetes mellitus (OR: 2.27, 95% CI: 1.42, 3.63; P=0.001); and transfusion of pRBC in POD 0 (OR: 2.61, 95% CI: 1.26, 5.40; P=0.010).
Discussion
This retrospective study demonstrated that perioperative sedative use is not associated with AKI in POD 0–3 after TKA or THA under spinal anesthesia. In particular, the use of propofol or dexmedetomidine, which we hypothesized would decrease the incidence of AKI, was not associated with AKI incidence. This study is valuable as it shows for the first time that, at least, sedative use in in postoperative AKI does not require caution.
A possible explanation that could be considered first for the lack of association between propofol or dexmedetomidine use and AKI incidence is the degree of renal ischemia or inflammatory reaction that can be induced by TKA or THA itself. Studies that reported propofol or dexmedetomidine decreases postoperative AKI are studies on cardiac surgery (10,11). Cardiopulmonary bypass is used in many cases during cardiac surgery, and in such cases, numerous cytokines are secreted, leading to severe renal ischemia (17). Indeed, AKI after cardiac surgery is common, with reported incidence of up to 50% (18). TKA or THA is limited to the lower limb, and by nature, stress response to TKA or THA is relatively lower than that to cardiac surgery. Thus, a lack of significant association between propofol or dexmedetomidine use and postoperative AKI may be attributed to the surgical characteristics of THA or TKA and cardiac surgery.
The second point to consider is that this study only included patients who received spinal anesthesia. Possibly, stimulation from surgical stress may have been lower with spinal anesthesia than in with general anesthesia. Considering that inflammatory reaction is known to be closely associated with occurrence of AKI (19), the anti-inflammatory effect of propofol or dexmedetomidine could have been lessened during spinal anesthesia.
Interestingly, this study reported factors shown to be associated with increased AKI after TKA or THA. First, the factor that was most strongly associated with increased AKI was preoperative chronic kidney disease (CKD) status, which can be determined through preoperative eGFR. This result is consistent with the recently reported findings (4,20), in one of which Weistein et al. reported that preoperative compromised renal function is the best predictor of increased risk of AKI (4). In the study by Weinstein et al., baseline eGFR was simply divided into two categories: >60 and ≤60 mL/min/1.73 m2. When the baseline eGFR was ≤ 60, the risk of AKI increased 2.484 folds (95% CI: 1.195, 4.994, P=0.012). In contrast, the patients in our study were divided into more groups according to their preoperative eGFR status, and we presented OR for each AKI for preoperative eGFR 30–60 (CKD stage 3) and eGFR <30 or receiving preoperative chronic RRT (CKD stage 4). This showed that patients with stage 4 CKD, including end-stage renal disease, had the greatest increase in risk of AKI. While CKD is a well-known predisposing factor for AKI (21), the findings of this study showed concretely how much AKI risk is increased after THA or TKA for each CKD stage.
Another point to consider is the incidence rate of AKI (2.6%) reported in this study after TKA or THA. This incidence rate is higher than the 1.1% reported by Gharaibeh et al. or the 1.85% reported by Weinstein et al., but lower than 3.3% reported by Kuan-Ting Wu et al. or the 6.8% reported by Jiang et al. (3-5,20). The incidence of AKI is largely affected by the level of the health care institution; hence, it could vary greatly across countries (22). Thus, when interpreting study results on AKI after THA or TKA, the study location must be taken into consideration. Our study showed an AKI incidence rate of 2.6% in POD 0-3 after TKA and THA, which is significant in that this is the first report of AKI incidence after TKA and THA in South Korea.
This study had some limitations. First, given the retrospective observational design of this study, data quality and accuracy are naturally limited. Second, the generalizability of this study is limited, as this was a single-center study. Finally, varying duration of urinary catheterization between patients was taken into consideration and only serum creatinine was used for the diagnosis of AKI in an effort to have accurate AKI diagnosis. Thus, sensitivity for diagnosis of AKI could be low. Nonetheless, this study is significant as it is the first to show that sedative use, such as propofol and dexmedetomidine, is not associated with decreased risk of AKI after TKA or THA.
In conclusion, this study demonstrated no significant association between sedative uses (propofol and dexmedetomidine) and incidence of AKI after THA or TKA under spinal anesthesia, and use of such sedatives does not require extreme caution.
Acknowledgments
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was conducted after approval by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (SNUBH) (Approval Number: B-1711/432-103). The requirement to obtain informed consent was waived by the IRB because of the retrospective nature of the study.
References
- Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am 2015;97:1386-97. [Crossref] [PubMed]
- Soffin EM. YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth 2016;117:iii62-72. [Crossref] [PubMed]
- Jiang EX, Gogineni HC, Mayerson JL, et al. Acute Kidney Disease After Total Hip and Knee Arthroplasty: Incidence and Associated Factors. J Arthroplasty 2017;32:2381-5. [Crossref] [PubMed]
- Weinstein SM. YaDeau JT, Memtsoudis SG. Lack of Association Between Levels and Length of Intraoperative Controlled Hypotension and Acute Kidney Injury in Total Hip Arthroplasty Patients Receiving Neuraxial Anesthesia. Reg Anesth Pain Med 2018;43:725-31. [PubMed]
- Wu KT, Chen CY, Chen B, et al. The Incidence and Risk Factors of Acute Kidney Disease after Total Knee Arthroplasty with Early Postoperative Volume Supplement. Biomed Res Int 2018;2018:8718545. [Crossref] [PubMed]
- Siddiqi A, White PB, Etcheson JI, et al. Acute Kidney Injury after Total Knee Arthroplasty: A Clinical Review. Surg Technol Int 2017;31:243-52. [PubMed]
- Turnbull ZA, Sastow D, Giambrone GP, et al. Anesthesia for the patient undergoing total knee replacement: current status and future prospects. Local Reg Anesth 2017;10:1-7. [Crossref] [PubMed]
- Hohener D, Blumenthal S, Borgeat A. Sedation and regional anaesthesia in the adult patient. Br J Anaesth 2008;100:8-16. [Crossref] [PubMed]
- Leite TT, Macedo E, Martins Ida S, et al. Renal Outcomes in Critically Ill Patients Receiving Propofol or Midazolam. Clin J Am Soc Nephrol 2015;10:1937-45. [Crossref] [PubMed]
- Yoo YC, Shim JK, Song Y, et al. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int 2014;86:414-22. [Crossref] [PubMed]
- Liu Y, Sheng B, Wang S, et al. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018;18:7. [Crossref] [PubMed]
- Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care 2011;15:R153. [Crossref] [PubMed]
- Tan F, Chen Y, Yuan D, et al. Dexmedetomidine protects against acute kidney injury through downregulating inflammatory reactions in endotoxemia rats. Biomed Rep 2015;3:365-70. [Crossref] [PubMed]
- Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Hallan S, Asberg A, Lindberg M, et al. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis 2004;44:84-93. [Crossref] [PubMed]
- O'Neal JB, Shaw AD. Billings FTt. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 2016;20:187. [Crossref] [PubMed]
- Lagny MG, Jouret F, Koch JN, et al. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol 2015;16:76. [Crossref] [PubMed]
- Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol 2016;27:371-9. [Crossref] [PubMed]
- Gharaibeh KA, Hamadah AM, Sierra RJ, et al. The Rate of Acute Kidney Injury After Total Hip Arthroplasty Is Low but Increases Significantly in Patients with Specific Comorbidities. J Bone Joint Surg Am 2017;99:1819-26. [PubMed]
- Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58-66. [Crossref] [PubMed]
- Cerda J, Mohan S, Garcia-Garcia G, et al. Acute Kidney Injury Recognition in Low- and Middle-Income Countries. Kidney Int Rep 2017;2:530-43. [Crossref] [PubMed]