
Extracellular vesicles in hepatocellular cancer and cholangiocarcinoma
Introduction
Extracellular vesicles (EVs) are subcellular components produced by a variety of cells, which are microscopic spherical particles containing specific lipids, RNA species, DNA and proteins (1). These small particles of 40 to 5,000 nm in diameter are released by several cells into the extracellular matrix. While they were previously considered as a means to discard cellular metabolic waste, recently emerging evidence suggest that they are essential players in cell-to-cell communication (2,3). Based on their cellular biogenesis and characteristics, EVs are classified into three categories: exosomes, microvesicles, and apoptotic bodies. Exosomes, small vesicles (40–100 nm) synthesized and matured inside multivesicular bodies, are the most studied EVs (4). Also, atypically large (1–10 µm diameter) vesicles, termed large oncosomes, can be secreted by specific cancer cells (5). Given the fact that EVs and their cargo represent the physiopathological state of the cells by which they are emitted, and cancer cells produce a relatively large amount of EVs, recent studies have suggested that EVs and their cargo have a significant impact on the tumor microenvironment, tumor growth and differentiation (6). There is an increasing amount of research suggesting that EVs and their cargo serve as promising biomarker candidates in the diagnosis and prognosis of cancers (7,8). Furthermore, these particles wrapped in lipid bilayer represent a potential target for therapeutic use, and ex vivo modified or synthesized EVs can be engineered as therapeutic shuttles in treating cancerous diseases (9).
Hepatocellular carcinoma (HCC) is the most common primary liver cancer which is identified as the second leading cause of all cancer-related deaths (10,11). Due to the absence of typical early clinical manifestations and insufficiency of public surveillance (serum AFP test and abdominal ultrasound), many patients with liver cancer have lost the chance of radical surgical resection by the time of diagnosis (12). Localized interventional chemoembolization, systemic therapy, and chemotherapy can only prolong the survival time of patients with advanced liver cancer for a rather short period (10,12). The same dilemma goes with cholangiocarcinoma (CCA), let alone the worse prognosis of CCA is another problem we have to cope with (12-14). Studies have found that EVs play a critical role in HCC and CCA carcinogenesis and metastasis. Altered EVs in serum and bile as well as their cargo may serve as diagnostic biomarkers and therapeutic target for HCC and CCA and engineered EVs may be a brand-new therapeutic approach (15-17). Here we reviewed the research progress of EVs and their cargo in the diagnosis and treatment of HCC and CCA.
Liver-derived EVs and their physiological characteristics
The liver is a multicellular substantive organ composed of hepatocytes, bile duct epitheliums, hepatic stellate cells (HSCs), sinusoidal endothelial cells, and various immune cells (18). To perform a normal liver function, cells within the liver need to collaborate according to intercellular exchanges of substances and information. In addition to direct contact between liver cells, liver cell-derived EVs are essential carriers of intrahepatic signal transduction (4). EVs released from different cells function distinctively. For example, EVs released by hepatocytes can regulate their proliferation, while HSCs-derived EVs are involved in liver fibrosis formation (19). When the liver is under stress or pathological conditions, EVs secreted by liver cells undergo significant changes in both quantity and quality, the concentration of EVs and the composition of EVs cargo, namely proteins, lipids, and nucleic acids, and etc., changes dramatically (20).
Bile synthesized by hepatocytes runs through biliary tract. It is a non-circulating fluid that contacts with the tumors directly, and it collects EVs released from hepatocytes, bile duct epitheliums, and cancerous cells, and etc. (1,21). EVs in bile are rich in microRNA (mi-RNA), long noncoding RNA (lncRNA) and proteins. They participate in the regulation of the biliary tract microenvironment and biliary cells proliferation (22). Masyuk et al. studied the physiological function of EVs in bile and argued that bile EVs could adhere to cholangiocyte cilia to inhibit the proliferation of bile duct epitheliums by decreasing the phosphorylated-to-total ERK1/2 ratio and promoting the expression of miR-15A (21). Wang et al. found that chicken bile EVs can enhance the proliferation of CD4+ and CD8+ T cells and activate intrahepatic monocytes in immune responses (23).
Liver-derived EVs participate in cirrhosis formation. In the process of liver cirrhosis, HSCs are the primary effector cells that secrete a large amount of insoluble collagen to facilitate fibrogenesis. Activation of HSC is the crucial step of liver cirrhosis. Chen et al. illustrated that during the activation of HSC, the concentration of TWIST1, a basic helix-loop-helix transcription factor, in HSC-derived EVs decreased, which suppressed the expression of miR-214 and indirectly accelerated the synthesis of connective tissue growth factor 2 (CCN2), which takes the key role in the CCN2-dependent fibrogenesis (24). Charrier et al. stated that activated HSCs could wrap CCN2 into secreted EVs, and those EVs transport CCN2 to other quiescent or activated HSCs to further promote hepatic fibrosis (25).
EVs and their cargo as potential biomarkers in HCC and CCA
The concentration and content of EVs released from cancer cells are significantly different from those released from non-cancerous cells (6), making EVs a new source of cancer biomarkers lately. Studies have demonstrated that the mi-RNA components in ovarian cancer and lung cancer cell-derived EVs are distinct from the mi-RNA in non-cancerous cell-derived EVs respectively, enabling them to be used as diagnostic biomarkers for ovarian cancer and lung cancer (26,27). In another study, serum glypican-1 (GPC-1) positive EVs can be used as biomarkers in discriminating between pancreatic cancer and benign pancreatic lesions with absolute sensitivity and specificity, and the concentration of serum GPC-1 positive EVs is correlated with the tumor burden and prognosis of pancreatic cancer patients (28).
EVs as biomarkers for HCC
The study of potential biomarkers of HCC has always been a heated issue in the field of cancer research (29,30). In 2007, Valadi et al. found that exosomes can carry abundant mRNAs and mi-RNAs to target cells and express corresponding proteins in target cells (31). Since then, a growing number of evidence shows that cancer cells-derived EVs contain multiple types of RNA with cancer specificity and can serve as potential biomarkers (Table 1).
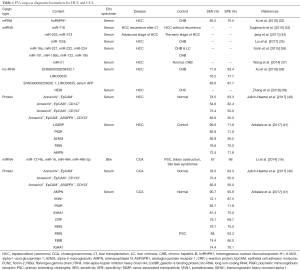
Full table
The predominant nucleotide content of HCC-derived EVs is various RNA species (17). Sohn et al. stated that the serum level of multiple EVs mi-RNAs in HCC patients was considerably higher than those in hepatitis B and liver cirrhosis patients, including miR-18a, miR-221, miR-222 and miR-224, yet miR-101, miR-106b, miR-122, and miR-195 were significantly lower (36). Sugimachi et al. found that compared with HCC patients who did not have cancer recurrence after liver transplantation, the patients suffering from cancer recurrence have a decreased expression of circulating miR-718 in serum EVs. The declining level of miR-718 was associated with HCC aggressiveness (33). Wang et al. claimed that the appearance of serum exosomal miR-21 in patients with HCC was significantly higher than that in healthy people and patients with hepatitis B. The expression of EVs miR-21 was correlated with the degree of liver cirrhosis and tumor stage (37).
The proportion of lnc-RNA in EVs RNA is rather small, accounting for about 3% of total EVs RNA (42). However, growing evidence has proven that EVs lnc-RNA is involved in proliferation, recurrence, metastasis, and resistance to hypoxia and chemotherapy in liver cancer. Kogure et al. indicated that 1198-bp lnc-RNA, termed as TUC339, was significantly upregulated in HCC cells. The proliferation and adhesion of HCC cells could be promoted with increasing expression of TUC339or inhibited with decreasing expression of TUC339 (43). Another study demonstrated that lnc-RNA H19 was enriched in EVs released from CD90+ liver cancer stem cells. lncRNA H19 induces an early recurrence of HCC and promotes metastasis of circulating CD90+ liver cancer stem cells by modulating endothelial cell, promoting cell-to-cell adhesion and angiogenesis (44). Takahashi et al. found that when HCC cells were exposed to antitumor drugs such as sorafenib, the stress-responsive lncVLDLR was highly expressed in HCC cells as well as within EVs. lnc-VLDLR in EVs can reduce chemotherapy-induced cell death. The expression of ATP-binding cassette, subfamily G member 2 (ABCG2) is suppressed, and the viability of cancer cells is reduced by silencing lnc-VLDLR (45). Also, lnc-ROR is also related to the resistance of liver cancer cells. The expression level of lnc-ROR in normal hepatocytes is considerably low, while it is notably enriched within EVs of HCC cells, resulting in resistance to chemotherapy. Silencing the lnc-ROR could improve the sensitivity of HCC cells to chemotherapy. Besides, lnc-ROR could also serve as a potential biomarker for HCC (46).
EVs as potential biomarkers for CCA
In CCA, Severino et al. found that bile EVs were significantly elevated in patients with CCA and pancreatic cancer. The median concentration of bile EVs from malignant patients was more than ten times higher than that from nonmalignant patients. It correctly classified malignant stenosis verse cholelithiasis and chronic pancreatitis (diagnostic accuracy, 100%). Results suggested that the diagnostic ability of bile EVs concentration was more superior when compared to the traditional serum carbohydrate antigen 19-9 (47).
Interestingly, there was no significant difference in serum EVs concentrations in patients with CCA, primary sclerosing cholangitis and HCC. However, several proteins with differential degrees of abundance were found in serum EV of CCA versus controls, some of which present significant diagnostic potential. In a comparison of patients with primary sclerosing cholangitis and HCC, patients with CCA suffered from higher levels of C-reactive protein, ficolins-2, fibrinogen γ chain, and plasma protease C1 inhibitors in serum EVs. Ficolin-2 and plasma protease C1 inhibitors were significantly elevated in the serum of patients with early staged CCA (I–II), indicating a higher diagnostic value than serum CA19-9 (41). Furthermore, a panel of mi-RNAs (191, 486-3p, 1274b, 16, 484) was found upregulated in bile EVs of patients with CCA comparing to patients with primary sclerosing cholangitis, biliary obstruction, and bile leak syndrome (16). Two lnc-RNAs (i.e., ENST00000588480.1 and ENST00000517758) were identified upregulated in the analysis of the bile EVs lnc-RNA profile in CCA patients versus patients with biliary obstruction (48) (Table 1).
EVs-based treatment
EVs can serve as therapeutic compounds carriers, such as chemicals, RNAs or proteins, and protect those compounds from enzymatic degradation. As a result, it bears ideal potential in the new drug-delivery system. Recent studies show that specific RNAs or chemotherapeutic drugs can be effectively delivered to tumor sites once they are packaged into EVs, leading to better pharmacokinetic efficiency and therapeutic efficacy of the drugs (49).
EVs-based treatment for HCC
On the one hand, EVs can be used as miRNAs delivery media in treating liver cancer. Stellate cell-derived EVs carrying miR-335-5p, a tumor suppressor miRNA downregulated in HCC, inhibit HCC cell viability in vitro and induce tumor shrinkage in vivo by suppressing proliferation and promote apoptosis (50). Moreover, miR-122 loaded EVs released from adipose tissue-derived mesenchymal stem cells (ADMSCs) increases HCC cell sensitivity to the chemotherapeutic agents, sorafenib and 5-FU, by inducing G0/G1 arrest and cell apoptosis in HCC cell cycle. Furthermore, intratumor injection of those loaded EVs in an HCC xenograft mouse model can strengthen the anti-tumor efficacy of sorafenib and reduce tumor size.
On the other hand, EVs are also used as toxic drugs delivery media in treating HCC. The same dosage of methotrexate via direct administration and microparticles-delivered administration causes 2% and 23% cell death respectively (51). Toxicated H22 cells-derived drug-encapsulating microparticles are also cytotoxic, triggering a domino-like cancer-killing effect. Methotrexate carried by microparticles is more effective than dissociative methotrexate in inhibiting tumor growth and prolonging survival time in xenograft mice (51).
EVs-based treatments of CCA
In CCA, fibroblast-derived EVs, carrying miR-195, inhibit CCA growth and invasiveness in vitro. miR-195 is an inhibitor of cancer growth and is generally downregulated in the CCA cells (9). Possible mechanisms of the anti-neoplastic effect of EVs carried miR-195 are downregulating VEGF, cell division control (CDC) proteins 25 and 42, as well as cyclin-dependent kinases (CDK) 1, 4, and 6.
Discussion
To date, the problem of lacking accurate early diagnosis and correct management for staged HCC and CCA is still seeking solutions. The carcinogenesis, development, and metastasis of HCC and CCA are somewhat complicated. Thus, the discovery of methods, as well as treatment for patients, remains a significant challenge. The presence of EVs, their tumor-associated cargo and their unique lipid bilayer characteristics in biological fluids make EVs excellent candidates for clinical application. Consequently, a growing number of studies have been conducted focusing on potential diagnostic use and innovative anti-tumor therapy via EVs. However, certain limitations remain to be unsolved: (I) the molecular mechanism of EVs synthesis, secretion, and participation in information exchange is not crystal clear; (II) although the International Society of Extracellular Vesicles (ISEV) provided official definition and subpopulation categories in 2012, recent literature suggested otherwise, such as large oncosomes. Types and characteristics of subpopulations of EVs need more in-depth study, not until then shall we have the terminology unified; (III) there is no universally recognized procedure in preparation, separation, purification, and reservation of the EVs, especially for each subpopulation.
In conclusion, EVs represent a rather appealing and promising field of research in HCC and CCA with multiple potential applications, including being diagnostic and prognostic biomarkers and new approaches for cancer treatment. However, more research and data are still needed to learn about EVs and their possible role in cancerous disease.
Acknowledgements
Funding: This study was supported by grants from the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1–001) and the National Natural Science Foundation of China (31500818).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066. [Crossref] [PubMed]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373-83. [Crossref] [PubMed]
- Maas SLN, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol 2017;27:172-88. [Crossref] [PubMed]
- Hirsova P, Ibrahim SH, Verma VK, et al. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016;64:2219-33. [Crossref] [PubMed]
- Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 2015;40:41-51. [Crossref] [PubMed]
- D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev 2012;26:1287-99. [Crossref] [PubMed]
- Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med 2018;379:958-66. [Crossref] [PubMed]
- Revenfeld AL, Baek R, Nielsen MH, et al. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther 2014;36:830-46. [Crossref] [PubMed]
- Li L, Piontek K, Ishida M, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017;65:501-14. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Sia D, Villanueva A, Friedman SL, et al. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017;152:745-61. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Giorgio A, Gatti P, Matteucci P, et al. Ablative therapies for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2018;7:192-4. [Crossref] [PubMed]
- Pawlik TM. Intrahepatic cholangiocarcinoma: from diagnosis to treatment. Hepatobiliary Surg Nutr 2017;6:1. [Crossref] [PubMed]
- Rao Q, Zuo B, Lu Z, et al. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology 2016;64:456-72. [Crossref] [PubMed]
- Li L, Masica D, Ishida M, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 2014;60:896-907. [Crossref] [PubMed]
- He M, Qin H, Poon TC, et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis 2015;36:1008-18. [Crossref] [PubMed]
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147-63. [Crossref] [PubMed]
- Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol 2013;59:621-5. [Crossref] [PubMed]
- Momen-Heravi F, Bala S, Kodys K, et al. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep 2015;5:9991. [Crossref] [PubMed]
- Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 2010;299:G990-9. [Crossref] [PubMed]
- Li L, Piontek KB, Kumbhari V, et al. Isolation and Profiling of MicroRNA-containing Exosomes from Human Bile. J Vis Exp 2016;(112).
- Wang Y, Wang G, Wang Z, et al. Chicken biliary exosomes enhance CD4(+)T proliferation and inhibit ALV-J replication in liver. Biochem Cell Biol 2014;92:145-51. [Crossref] [PubMed]
- Chen L, Chen R, Kemper S, et al. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol 2015;309:G491-9. [Crossref] [PubMed]
- Charrier A, Chen R, Chen L, et al. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery 2014;156:548-55. [Crossref] [PubMed]
- Rabinowits G, Gercel-Taylor C, Day JM, et al. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 2009;10:42-6. [Crossref] [PubMed]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008;110:13-21. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [Crossref] [PubMed]
- Bravi F, La Vecchia C, Turati F. Green tea and liver cancer. Hepatobiliary Surg Nutr 2017;6:127-9. [Crossref] [PubMed]
- Wang Y, Yang H, Xu H, et al. Golgi protein 73, not Glypican-3, may be a tumor marker complementary to alpha-Fetoprotein for hepatocellular carcinoma diagnosis. J Gastroenterol Hepatol 2014;29:597-602. [Crossref] [PubMed]
- Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Xu H, Dong X, Chen Y, et al. Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma. Clin Chem Lab Med 2018;56:479-84. [Crossref] [PubMed]
- Sugimachi K, Matsumura T, Hirata H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer 2015;112:532-8. [Crossref] [PubMed]
- Jang SY, Kim G, Hur K, et al. Circulating serum exosomal microRNA-203 as a non-invasive biomarker for predicting prognosis in hepatocellular carcinoma. Journal of Hepatology 2017;66:S672-3. [Crossref]
- Liu W, Hu J, Zhou K, et al. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Onco Targets Ther 2017;10:3843-51. [Crossref] [PubMed]
- Sohn W, Kim J, Kang SH, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med 2015;47:e184. [Crossref] [PubMed]
- Wang H, Hou L, Li A, et al. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int 2014;2014:864894. [PubMed]
- Xu H, Chen Y, Dong X, et al. Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev 2018;27:710-6. [Crossref] [PubMed]
- Zhang C, Yang X, Qi Q, et al. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark 2018;21:651-9. [Crossref] [PubMed]
- Julich-Haertel H, Urban SK, Krawczyk M, et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol 2017;67:282-92. [Crossref] [PubMed]
- Arbelaiz A, Azkargorta M, Krawczyk M, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017;66:1125-43. [Crossref] [PubMed]
- Gezer U, Ozgur E, Cetinkaya M, et al. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int 2014;38:1076-9. [PubMed]
- Kogure T, Yan IK, Lin WL, et al. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer 2013;4:261-72. [Crossref] [PubMed]
- Conigliaro A, Costa V, Lo Dico A, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer 2015;14:155. [Crossref] [PubMed]
- Takahashi K, Yan IK, Wood J, et al. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res 2014;12:1377-87. [Crossref] [PubMed]
- Takahashi K, Yan IK, Kogure T, et al. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 2014;4:458-67. [Crossref] [PubMed]
- Severino V, Dumonceau JM, Delhaye M, et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology 2017;153:495-504.e8. [Crossref] [PubMed]
- Ge X, Wang Y, Nie J, et al. The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget 2017;8:69995-70005. [Crossref] [PubMed]
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7:653-64. [Crossref] [PubMed]
- Wang F, Li L, Piontek K, et al. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 2018;67:940-54. [Crossref] [PubMed]
- Tang K, Zhang Y, Zhang H, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun 2012;3:1282. [Crossref] [PubMed]


