
Central arterial pressure predicts in-hospital major adverse cardiovascular events after acute ST-segment elevation myocardial infarction: a retrospective cohort study
Highlight box
Key findings
• Central systolic pressure (CSP), central diastolic pressure (CDP), and central mean pressure (CMP) have good predictive ability for postoperative in-hospital outcomes in ST-segment elevation myocardial infarction (STEMI) patients.
What is known and what is new?
• Central arterial pressure (CAP) is closely related to the pathophysiological process of cardiovascular disease, and its relationship with outcomes after percutaneous coronary intervention (PCI) in STEMI patients remains unclear.
• Higher (>137.5 mmHg) or lower (<102 mmHg) CSP, lower CDP (<61 mmHg), higher (>55 mmHg) or lower (<29 mmHg) central pulse pressure (CPP), and higher (>101 mmHg) or lower (<76 mmHg) CMP were independent risk factors for major adverse cardiovascular events (MACEs) after acute ST-segment elevation myocardial infarction.
What is the implication, and what should change now?
• Clinicians should observe these CAP indicators in PCI.
Introduction
Acute ST-segment elevated myocardial infarction (STEMI) is the most severe type of acute coronary syndrome. Although emergency percutaneous coronary intervention (PCI) is the most effective way to save the ischemic myocardium, there are many prognostic factors, including preoperative blood pressure, vascular patency time, underlying diseases, age, hypertension, diabetes, and criminal vessels. It is generally believed that brachial systolic and diastolic blood pressures have a “J” or “U”-shaped relationship with mortality in patients with acute coronary syndrome (1-4). However, there are few studies on the relationship between central arterial pressure (CAP) and outcomes after PCI, and there is no generally accepted reference range and diagnostic threshold (5). CAP refers to the lateral pressure on the vessel wall at the root of the ascending aorta (6). The root of the ascending aorta is close to the heart and coronary arteries, and CAP can directly reflect the hemodynamic status of this region. In recent years, studies have suggested that CAP has better predictive value than peripheral arterial pressure in the occurrence, development, and clinical events of cardiovascular disease (7-10). Moreover, hemodynamics is more complex in acute myocardial infarction (AMI), and it is more difficult to interpret CAP and cardiac function from the peripheral arterial pressure. At present, emergency coronary intervention has been popularized worldwide. CAP can be accurately measured at the same time as the interventional procedure. The aim of this study was to evaluate and compare the relationship between CAP measured at the beginning of PCI and outcomes during postoperative hospitalization. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1079/rc).
Methods
Study population
This was a retrospective cohort study including STEMI patients who were admitted to Beijing Aerospace General Hospital for emergency PCI from December 2014 to March 2021. The diagnosis of STEMI is based on (11): (I) symptoms of acute myocardial ischemia; (II) increased cardiac troponin T (cTnI) value or dynamic changes were detected, and at least 1 value was higher than the upper limit of 99% of the normal value; (III) electrocardiogram: new ST segment elevation at J point in 2 adjacent leads, among which, V2–V3 leads ≥2.5 mm (male, <40 years old); ≥2 mm (male, ≥40 years old); ≥1.5 mm (female, regardless of age); other leads ≥1.0 mm. The inclusion criteria were as follows: (I) age ≥18 years; (II) underwent coronary intervention; (III) meeting the diagnostic criteria for STEMI; (IV) hospitalization time ≥1 day. The exclusion criteria were as follows: (I) malignant tumor; (II) active rheumatism; (III) aortic dissection; (IV) intra-aortic balloon pulsation was implanted before PCI; (V) pacemaker was implanted before PCI. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Aerospace General Hospital (approval No. 2021-Clinic-1), and informed consent was taken from all the patients.
Data collection
All baseline clinical data were collected from electronical medical record.
Blood test
Blood was collected from all patients through the cubital vein, and high-sensitivity cardiac troponin I (hs-cTnI), B-type natriuretic peptide (BNP), and creatinine (Cr) were tested before PCI. On the second day after PCI, the fasting total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and glycosylated hemoglobin (HbA1c) were tested. Echocardiography (MINDRAY M8; Shenzhen, China) was performed daily, and hs-cTnI peak value within 72 hours and BNP peak value within 72 hours were recorded.
In-hospital complication
Clinical data were collected from the in-hospital electronic health information system (HIS). In-hospital outcomes, including cardiac death, acute heart failure, cardiogenic shock, malignant arrhythmias, and AMI, were recorded. Relevant outcomes were defined as follows: (I) Heart failure (HF): The patient has clinical manifestations such as dyspnea, bilateral wet rales, or wheezing, and chest X-ray shows pulmonary congestion and/or pulmonary edema, Killip grade 2–3. (II) Cardiac shock (CS): low blood pressure [systolic blood pressure (SBP) <90 mmHg] in the presence of sufficient blood volume, urine output <0.5 mL/kg·h accompanied by tissue hypoperfusion, clammy limbs, consciousness status change, blood lactate >2 mmol/L, metabolic acidosis (pH<7.35), Killip grade 4. (III) Malignant arrhythmia (MA): pre-excitation syndrome with atrial fibrillation, ventricular flutter, ventricular fibrillation, sustained ventricular tachycardia, torsades de pointes (TdP), sick sinus syndrome, type II AV block, third-degree or high-grade AV block. (IV) Cardiac death (CD): including (i) cardiac arrest, which refers to the sudden stop of the left ventricular ejection function of the heart, resulting in cessation of blood circulation, respiratory arrest, and loss of consciousness; (ii) sudden cardiac death refers to cardiogenic spontaneous death characterized by loss of consciousness within 1 hour after the occurrence of acute symptoms. (V) AMI: postoperative angina pectoris with re-elevated myocardial enzymes.
Coronary intervention and CAP assessment
Coronary angiography was performed through the radial artery, and the left and right coronary arteries were evaluated from at least 2–4 positions. The angiography results were jointly judged by 2 cardiologists. Treatments such as thrombus aspiration, stent placement, and balloon dilation were decided according to the actual situation. During CAP measurement, a 5F pig tail catheter was inserted through the radial artery, the head end of the catheter was placed 1–2 cm above the ascending aortic valve, and the tail end was connected to a multi-channel physiological recorder (TRAM-RAC 4A; GE Healthcare, Chicago, IL, USA) through a transducer, simultaneously accompanied by multiple flushes with heparinized saline, zeroing at the midline of the axilla. After obtaining a stable pressure waveform, the invasive central systolic pressure (CSP) and central diastolic pressure (CDP) were obtained by taking the average value. Central pulse pressure (CPP) = CSP − CDP; central mean pressure (CMP) = CDP + CPP × 1/3. Before emergency PCI, a 300 mg loading dose of aspirin was given, and a loading dose of ticagrelor 180 mg or clopidogrel 600 mg was given at the same time.
Statistical analysis
The statistical software SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R3.4.0 (R Foundation for statistical Computing, Vienna, Austria) were used for statistical processing. The continuous data were expressed as mean ± standard deviation. The BNP value was in line with the normal distribution after logarithmic transformation (LnBNP). One-way analysis of variance (ANOVA) was used to compare the means between the 2 groups. Categorical data were expressed as percentages, and rates were compared using the χ2 test. Pearson's correlation test was used for correlation analysis between groups. Binomial logistic regression was used for multivariate analysis, and the variables included age, gender, heart rate, history of hypertension, history of intervention or bypass, history of myocardial infarction, anterior myocardial infarction, preoperative thrombolysis in myocardial infarction (TIMI) flow, estimated glomerular filtration rate (eGFR), preoperative BNP and hs-cTnI, postoperative BNP and hs-cTnI, left ventricular ejection fraction (LVEF), and preoperative medication history. The predictive ability of blood pressure indicators was compared using area under the receiver operating characteristic (ROC) curve (AUC) and multivariate logistic regression-based net reclassification index (NRI) and integrated discrimination improvement (IDI). A 4-node restricted cubic spline (RCS) plot based on multivariate logistic regression was used to reflect the potential nonlinear relationship between CAP indicators and the occurrence of major adverse cardiovascular events (MACEs). A two-sided P<0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 512 STEMI patients were included. According to the occurrence of outcomes, cases were divided into 2 groups, a MACEs group and a non-MACEs group (Table 1). Comparing the 2 groups of patients, the ratio of males, age, heart rate, BNP, and cTnI at admission, and peak BNP in the MACEs group were significantly higher than those in the non-MACEs group, whereas HDL-C level, LVEF, the ratio of taking antiplatelet drugs, and the ratio of taking statins were lower than those in the non-MACEs group, and the difference was statistically significant (P<0.05).
Table 1
| Variables | General population (n=512) | MACEs group (n=116) | Non-MACEs group (n=396) | F/χ2 | P value |
|---|---|---|---|---|---|
| Age (years) | 61.53±14.0 | 68.35±13.90 | 59.53±13.42 | 38.14 | 0.000 |
| Male | 412 | 83 (71.6) | 329 (83.1) | 7.59 | 0.006 |
| Heart rate (beat/minutes) | 76.5±17.9 | 80.32±21.71 | 75.38±16.54 | 6.88 | 0.009 |
| Diabetes | 159 | 35 (30.2) | 124 (31.3) | 0.06 | 0.815 |
| Hypertension | 291 | 79 (68.1) | 212 (53.5) | 7.76 | 0.005 |
| Smoking | 228 | 46 (39.7) | 182 (46.0) | 1.44 | 0.230 |
| Prior PCI of CABG | 60 | 21 (18.1) | 39 (9.8) | 5.91 | 0.015 |
| Prior MI | 29 | 10 (8.6) | 19 (4.8) | 2.45 | 0.117 |
| Anterior wall MI | 249 | 69 (59.5) | 180 (45.4) | 7.07 | 0.008 |
| Multivessel lesion | 462 | 107 (92.2) | 355 (89.6) | 0.69 | 0.408 |
| TIMI flow ≥ grade 2 | 48 | 8 (6.9) | 40 (10.1) | 1.08 | 0.298 |
| Stent ≥2 | 136 | 25 (21.5) | 111 (28.0) | 1.93 | 0.165 |
| eGFR (mL/min/1.73 m2) | 125.8±41.6 | 112.03±42.69 | 129.82±40.43 | 16.93 | 0.000 |
| On admission | |||||
| LnBNP | 5.24±1.76 | 6.23±2.06 | 4.95±1.56 | 51.86 | 0.000 |
| cTnI (ng/mL) | 3.66±8.78 | 5.66±3.07 | 3.07±7.95 | 7.88 | 0.005 |
| Peak LnBNP | 7.28±1.24 | 8.32±1.25 | 6.98±1.06 | 132.20 | 0.000 |
| Peak cTnI (ng/mL) | 33.35±17.86 | 36.20±17.45 | 32.52±17.92 | 3.83 | 0.051 |
| LVEF | 59.9±10.2 | 52.89±13.05 | 61.98±8.22 | 81.72 | 0.000 |
| HbA1c | 6.8±2.88 | 7.07±5.21 | 6.75±1.68 | 1.11 | 0.292 |
| LDL-C (mmol/L) | 2.98±0.93 | 2.96±1.00 | 2.98±0.92 | 0.05 | 0.827 |
| HDL-C (mmol/L) | 1.13±2.21 | 1.49±4.57 | 1.02±0.42 | 4.05 | 0.045 |
| Antiplatelet | 77 | 27 (23.3) | 50 (12.6) | 7.96 | 0.005 |
| Statins | 91 | 28 (24.1) | 63 (15.9) | 4.16 | 0.041 |
| ACEI/ARB | 52 | 14 (12.1) | 38 (9.6) | 0.60 | 0.438 |
| βRB | 28 | 10 (8.6) | 18 (4.5) | 2.88 | 0.090 |
Data were expressed as n (%) or mean ± standard deviation. MACEs, major adverse cardiovascular events; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; MI, myocardial infarction; TIMI, thrombolysis in myocardial infarction; eGFR, estimated glomerular filtration rate; LnBNP, brain natriuretic peptide with logarithmic transformation; cTnI, cardiac troponin T; LVEF, left ventricular ejection fraction; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ACEI/ARB, angiotensin converts enzyme inhibitor/angiotensin receptor blocker; βRB, β receptor blocker.
In-hospital outcomes
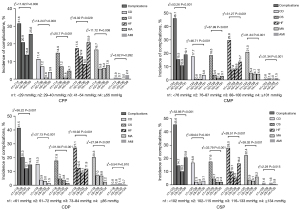
CSP, CDP, CPP, and CMP were each divided into 4 groups using the quartile method. Among the 512 patients, 116 experienced at least 1 in-hospital MACE, with an incidence rate of 22.60%, including 29 cardiac deaths (5.66%); cardiogenic shock in 34 cases (6.64%); heart failure 76 cases (14.84%); 45 cases (8.79%) of malignant arrhythmia; 5 cases (1.0%) of acute myocardial infarction. The incidence of in-hospital outcomes, cardiac death, cardiogenic shock, acute left heart failure and malignant arrhythmia in the 4 groups of blood pressure were significantly different (P<0.05). Among them, compared with the incidence of in-hospital outcomes in group 3 (n3), the low pressure subgroup of CSP had the highest relative risk (RR =4.46), followed by the low pressure subgroup of CMP (RR =4.22), the low pressure subgroup of CDP (RR =3.4), and the low pressure subgroup of CPP was the lowest (RR =2.29) (Figure 1).
Multivariate logistic regression analysis
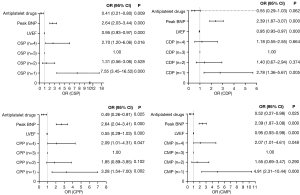
As displayed in Figure 2, the results of binomial multivariate logistic regression revealed that CSP n1 [<102 mmHg, OR =7.55, 95% confidence interval (CI): 3.45–16.52] and n4 (≥134 mmHg, OR =2.70, 95% CI: 1.20–6.06), CDP n1 (<61 mmHg, OR =2.78, 95% CI: 1.36–5.67), CPP n1 (<29 mmHg, OR =3.28, 95% CI: 1.54–7.00) and n4 (≥55 mmHg, OR =2.09, 95% CI: 1.01–4.31), CMP n1 (<76 mmHg, OR =4.91, 95% CI: 2.31–10.44) and n4 (≥101 mmHg, OR =2.07, 95% CI: 1.01–4.61), and elevated peak BNP were independent risk factors for in-hospital outcomes [odds ratio (OR) >2.0, P<0.05], and high LVEF value and history of antiplatelet drugs were protective factors (OR <1.0, P<0.05).
Relationship between CAP and in-hospital outcomes
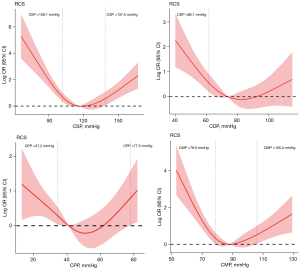
In order to further analyze the relationship between CAP and in-hospital outcomes, restricted cubic bar plots of CSP, CDP, CPP, and CMP were drawn to describe the nonlinear relationship between blood pressure and in-hospital outcomes (regression coefficient β). When CSP <108.1 mmHg or CSP >137.5 mmHg, the regression coefficient >0 (P<0.05); when CDP <66.1 mmHg, the regression coefficient >0 (P<0.05); when CPP <37.2 mmHg or CPP >77.5 mmHg, the regression coefficient >0 (P<0.05); the regression coefficient >0 (P<0.05) when CMP <78.9 mmHg or CMP >105.3 mmHg. The relationship between CSP, CMP, and in-hospital outcomes showed a “J” shape, the relationship between CPP and in-hospital outcomes showed a “U” shape, and the relationship between CDP and in-hospital outcomes showed an “L” shape (Figure 3).
Predictive value of CAP for in-hospital outcomes
The AUC was compared to reflect the ability of different CAP indicators to predict outcomes during hospitalization. CSP, CDP, CMP, and CPP all had an AUC over 0.5. There was no significant difference in the AUC between CSP, CDP, and CMP (P>0.05), but there was a significant difference between these 3 indicators and CPP (P<0.05), as shown in Figure 4. The pairwise NRI and IDI results of the 4 groups of CAP indicators (Table 2) showed that the predictive ability of CSP, CDP, and CMP was not significantly improved, and the difference was not statistically significant (P>0.05), but the 3 were significantly different from CPP. The prediction ability was improved, and the difference was statistically significant (P<0.05).
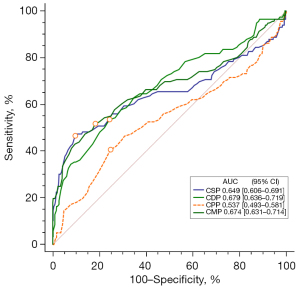
Table 2
| CAP | NRI | IDI | |||
|---|---|---|---|---|---|
| NRI (95% CI) | P value | IDI (95% CI) | P value | ||
| CDP/CSP | 0.1334 (−0.0732 to 0.3400) | 0.205 | 0.0051 (−0.0049 to 0.0151) | 0.314 | |
| CMP/CSP | 0.1872 (−0.0189 to 0.3933) | 0.075 | 0.0064 (4e-04 to 0.0124) | 0.037 | |
| CMP/CDP | 0.0408 (−0.1655 to 0.2470) | 0.698 | 0.0013 (−0.0039 to 0.0064) | 0.624 | |
| CSP/CPP | 0.3272 (0.1252 to 0.5293) | 0.001 | 0.0156 (0.0037 to 0.0275) | 0.010 | |
| CDP/CPP | 0.3365 (0.1358 to 0.5371) | 0.001 | 0.0207 (0.0056 to 0.0358) | 0.007 | |
| CMP/CPP | 0.3424 (0.1404 to 0.5444) | 0.001 | 0.0220 (0.0068 to 0.0371) | 0.004 | |
NRI, net reclassification index; IDI, integrated discrimination improvement; CAP, central arterial pressure; CDP, central diastolic pressure; CSP, central systolic pressure; CMP, central mean pressure; CPP, central pulse pressure; CI, confidence interval.
Discussion
Through retrospective analysis, this study found that among the central arterial pressures, higher (>137.5 mmHg) or lower (<102 mmHg) systolic CAP, lower (<61 mmHg) diastolic CAP, higher (>55 mmHg) or lower (<29 mmHg) pulse CAP, and higher (>101 mmHg) or lower (<76 mmHg) mean CAP were independent risk factors for MACEs. The relationship between systolic CAP and mean CAP and in-hospital outcomes showed a “J”-shaped relationship, diastolic CAP and in-hospital outcomes showed an “L”-shaped relationship, and pulse CAP and in-hospital outcomes showed a “U”-shaped relationship. There was no statistical difference detected between systolic CAP, diastolic CAP, and mean CAP in the prediction ability of in-hospital outcomes (P>0.05), but the comparison of the 3 with pulse CAP was statistically significant (P<0.05).
The CSP reflects the cardiac output and left ventricular afterload. When the CSP is low, the cardiac output is reduced, and the perfusion of the main organs is prone to be insufficient. Several studies have shown that, compared with peripheral arterial systolic blood pressure, CSP is not only more strongly correlated with coronary artery stenosis, but also more accurate in predicting the recurrence of acute coronary syndrome and cardiac death after PCI (12-14). Impaired LVEF and decreased SBP are important risk factors for heart failure and cardiac death (15-18). In AMI thrombolytic risk scores and other prognostic models, low admission SBP is considered strongly associated with mortality, and the relationship between SBP and MACEs is “J” shaped (19-21). Our study showed that the relationship between CSP and in-hospital outcomes was also in a “J” shape. The low-CSP group had the highest incidence of in-hospital outcomes (45.6%, P<0.05). After multivariate adjustment, the OR value of the low CSP group was significantly higher than that of the high CSP group (7.55 vs. 2.70). As CSP increased, CSP was positively correlated with LVEF (r=0.189, P<0.001). However, the incidence of cardiogenic shock, cardiac death, and malignant arrhythmias did not increase significantly. This is related to increased CSP, which increases left ventricular afterload, but also increases blood perfusion in major organs.
The European Society of Cardiology (ESC) guidelines recommend starting beta-blockers and angiotensin converting enzyme inhibitors (ACEI) within 24 hours of after stabilization of heart failure or in patients with LVEF <40% (11). However, in the ISIS-4 trial (22) and CONESENSUS-2 trial (23), the incidence of hypotension in the ACEI group was significantly higher than that in the placebo group (10.3% vs. 4.8%, 12% vs. 3%), and in some patients, the hypotension even caused discontinuation of ACEI therapy. At present, whether patients with low SBP are over-treated is gradually becoming increasingly investigated (24). Based on clinical trial evidence, ACEIs should not be used when peripheral SBP is <100 mmHg or 30 mmHg below baseline, especially in elderly patients. In this study, there was an independent and strong association between low CSP and cardiovascular mortality in patients with STEMI, and the results of the analysis showed that the risk of in-hospital outcomes increased gradually when CSP was <108.1 mmHg. When CSP >137.5 mmHg, it is necessary to consider starting antihypertensive drugs treatment to reduce the occurrence of acute left heart failure, for which the first choice of drugs is β-blockers and ACEI.
Coronary perfusion occurs during diastole, and diastolic blood pressure (DBP) is the main determinant of coronary blood flow. McEvoy (25) and Tsujimoto (26) demonstrated that low DBP (<60 mmHg, P<0.05) was associated with an increased risk of cardiovascular events and death in patients compared with DBP of 80–89 mmHg. In the international verapamil SP/trandolapril study (INVEST), among patients with a history of coronary revascularization (≥1 month), when DBP <70 mmHg, all-cause mortality and myocardial infarction rates increased 200%, when DBP <60 mmHg, the rates doubled, and there was a “J”-shaped relationship between the occurrence of MACEs and peripheral DBP, but the above were all long-term prognostic studies for peripheral DBP , and cannot directly and accurately reflect coronary perfusion (2). In our study, after adjusting for age, heart rate, and other confounding factors, only the low CDP group (<61 mmHg) was an independent risk factor for in-hospital outcomes (OR =2.03, P=0.003). The RCS analysis results showed that the relationship between CDP and in-hospital outcomes showed an “L”-shaped relationship, and the risk of in-hospital outcomes increased gradually when CDP <66.1 mmHg (P<0.05). When the lowest group (73–84 mmHg) was compared with the high CDP group (85mmHg), the incidence of in-hospital outcomes and acute left heart failure did not increase, and the difference was not statistically significant (P>0.05). Moreover, the incidence of cardiogenic death, cardiogenic shock, and malignant arrhythmia in the high CDP group was the lowest among the 4 groups. This may be because the main contradiction of STEMI patients is severe blood supply imbalance. Increasing CDP can directly increase coronary perfusion, significantly improve myocardial ischemia, and reduce postoperative outcomes.
In addition, in our study, the ability of central mean arterial pressure to predict in-hospital outcomes was comparable to that of SBP, and the nonlinear curve also showed a “J”-shaped relationship. CMP <78.9 mmHg and CMP >105.3 mmHg were shown to increase the risk of in-hospital complication. CMP primarily reflects perfusion of major organs, so in the absence of large randomized controlled trials, current American Heart Association/American College of Cardiology (AHA/ACC) guidelines recommend the use of inotropes and vasopressors to maintain systemic perfusion in acute myocardial infarction patients with low mean arterial pressure and organ function (class IIb) (27). Although several studies in recent years have shown that CPP is closely related to the long-term survival rate of patients with acute coronary syndrome, the relationship between the 2 factors is “J” shaped (28-31). In our study, we found that the relationship between CCP and in-hospital outcomes showed a “U”-shaped relationship, which was consistent with the findings of Ndrepepa et al. (32). However, among the 4 CAP indicators, CPP has the lowest ability to predict in-hospital outcomes. The possible reason is that CPP mainly reflects the compliance of the aorta and is related to the long-term prognosis of cardiovascular disease, whereas the short-term prognosis of patients with STEMI is more closely related to hemodynamic changes.
There were some limitations of this study: First, it was a retrospective study, and some patients were excluded due to incomplete data, which may have caused a certain bias in case selection; second, this was a single-center study, and there may have been some differences of intervention strategy for patients when compared with other medical centers, and the short-term prognosis of patients may be affected; thirdly, this study did not follow up the patients for a prolonged period, and it was impossible to analyze the effect of preoperative central arterial blood pressure on the long-term prognosis.
Conclusions
When STEMI occurs, low CSP is most closely related to in-hospital outcomes, followed by low CMP, low CDP, and low CPP has the weakest correlation. Although high CSP, high CMP, and high CPP are also unfavorable for in-hospital outcomes, high CDP does not appear to be significantly harmful.
Acknowledgments
Funding: This study was supported by the Capital Medical University Natural Cultivation Fund (No. 1200020106) and National Natural Science Foundation of China (No. 1641072).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1079/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1079/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1079/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1079/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Aerospace General Hospital (approval No. 2021-Clinic-1), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bangalore S, Qin J, Sloan S, et al. What is the optimal blood pressure in patients after acute coronary syndromes?: Relationship of blood pressure and cardiovascular events in the PRavastatin OR atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction (PROVE IT-TIMI) 22 trial. Circulation 2010;122:2142-51. [Crossref] [PubMed]
- Denardo SJ, Messerli FH, Gaxiola E, et al. Coronary revascularization strategy and outcomes according to blood pressure (from the International Verapamil SR-Trandolapril Study [INVEST]). Am J Cardiol 2010;106:498-503. [Crossref] [PubMed]
- Wei H, Hongwei L, Ying S, et al. The U-shape relationship between pulse pressure level on inpatient admission and long-term mortality in acute coronary syndrome patients undergoing percutaneous coronary intervention. J Clin Hypertens (Greenwich) 2022;24:58-66. [Crossref] [PubMed]
- Tang Y, Liu S, Shi Y, et al. Association of blood pressure in the first-week of hospitalization and long-term mortality in patients with acute left ventricular myocardial infarction. Int J Cardiol 2022;349:18-26. [Crossref] [PubMed]
- Kuznetsov AA, Tsvetkova EE, Denisova DV, et al. Central Aortic Pressure: Reference and Diagnostic Values. Kardiologiia 2019;59:11-7. [Crossref] [PubMed]
- O'Rourke MF, Seward JB. Central arterial pressure and arterial pressure pulse: new views entering the second century after Korotkov. Mayo Clin Proc 2006;81:1057-68. [Crossref] [PubMed]
- Bednarek A, Jankowski P, Olszanecka A, et al. 24-hour central blood pressure and intermediate cardiovascular phenotypes in untreated subjects. Am J Cardiovasc Dis 2014;4:177-87. [PubMed]
- Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007;50:197-203. [Crossref] [PubMed]
- Omboni S, Arystan A, Benczur B. Ambulatory monitoring of central arterial pressure, wave reflections, and arterial stiffness in patients at cardiovascular risk. J Hum Hypertens 2022;36:352-63. [Crossref] [PubMed]
- Boczar KE, Boodhwani M, Beauchesne L, et al. Aortic Stiffness, Central Blood Pressure, and Pulsatile Arterial Load Predict Future Thoracic Aortic Aneurysm Expansion. Hypertension 2021;77:126-34. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Chirinos JA, Zambrano JP, Chakko S, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension 2005;45:980-5. [Crossref] [PubMed]
- Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004;109:184-9. [Crossref] [PubMed]
- Danchin N, Benetos A, Lopez-Sublet M, et al. Aortic pulse pressure is related to the presence and extent of coronary artery disease in men undergoing diagnostic coronary angiography: a multicenter study. Am J Hypertens 2004;17:129-33. [Crossref] [PubMed]
- Adamopoulos C, Zannad F, Fay R, et al. Ejection fraction and blood pressure are important and interactive predictors of 4-week mortality in severe acute heart failure. Eur J Heart Fail 2007;9:935-41. [Crossref] [PubMed]
- Ather S, Chan W, Chillar A, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J 2011;161:567-73. [Crossref] [PubMed]
- Bao J, Kan R, Chen J, et al. Combination pharmacotherapies for cardiac reverse remodeling in heart failure patients with reduced ejection fraction: A systematic review and network meta-analysis of randomized clinical trials. Pharmacol Res 2021;169:105573. [Crossref] [PubMed]
- Huang R, Lin Y, Liu M, et al. Time in Target Range for Systolic Blood Pressure and Cardiovascular Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc 2022;11:e022765. [Crossref] [PubMed]
- Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102:2031-7. [Crossref] [PubMed]
- Stebbins A, Mehta RH, Armstrong PW, et al. A model for predicting mortality in acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: results from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. Circ Cardiovasc Interv 2010;3:414-22. [Crossref] [PubMed]
- Chin CT, Chen AY, Wang TY, et al. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: the acute coronary treatment and intervention outcomes network (ACTION) registry-get with the guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J 2011;161:113-122.e2. [Crossref] [PubMed]
- ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet 1995;345:669-85. [Crossref] [PubMed]
- Swedberg K, Held P, Kjekshus J, et al. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). N Engl J Med 1992;327:678-84. [Crossref] [PubMed]
- Mouhat B, Putot A, Hanon O, et al. Low Systolic Blood Pressure and Mortality in Elderly Patients After Acute Myocardial Infarction. J Am Heart Assoc 2020;9:e013030. [Crossref] [PubMed]
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol 2016;68:1713-22. [Crossref] [PubMed]
- Tsujimoto T, Kajio H. Low diastolic blood pressure and adverse outcomes in heart failure with preserved ejection fraction. Int J Cardiol 2018;263:69-74. [Crossref] [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362-425. [Crossref] [PubMed]
- Wu HP, Lin MJ. Central aortic pressure and long-term outcome in hypertensive patients undergoing percutaneous coronary intervention. Sci Rep 2020;10:17420. [Crossref] [PubMed]
- Rosenwasser RF, Shah NK, Smith SM, et al. Baseline predictors of central aortic blood pressure: a PEAR substudy. J Am Soc Hypertens 2014;8:152-8. [Crossref] [PubMed]
- Vlachopoulos C, Aznaouridis K, O'Rourke MF, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010;31:1865-71. [Crossref] [PubMed]
- Selvaraj S, Steg PG, Elbez Y, et al. Pulse Pressure and Risk for Cardiovascular Events in Patients With Atherothrombosis: From the REACH Registry. J Am Coll Cardiol 2016;67:392-403. [Crossref] [PubMed]
- Ndrepepa G, Cassese S, Kufner S, et al. U-shaped association of central pulse pressure with long-term prognosis after ST-segment elevation myocardial infarction. Heart Vessels 2019;34:1104-12. [Crossref] [PubMed]
(English Language Editor: J. Jones)


