
Maternal obesity inhibits placental angiogenesis by down-regulating the SIRT1/PGC-1α pathway
Introduction
In recent years, the prevalence of obesity has increased markedly throughout the world (1-3). Pre-pregnancy obesity is a risk factor for complications during both pregnancy and delivery, including miscarriage, pre-eclampsia, gestational diabetes, fetal macrosomia, and cesarean section (4-7). The placenta, a transient organ, is important in the exchange of gases, nutrients, hormones, and waste products between mother and fetus. An adverse maternal environment, such as diet-induced obesity, impairs the function and alters the structure of the placenta (8). Maternal obesity has been demonstrated to induce ectopic lipid deposition in the placenta, leading to lipotoxicity and chronic inflammation (9,10). In this condition, placental development and angiogenesis are inhibited, resulting in placental dysfunction (11-14).
The placenta contains a complex vascular network, requiring extensive angiogenesis. The process of normal placentation is associated with the activity of various cytokines, such as vascular endothelial growth factor A (VEGFA), angiotensin I (Ang I), and angiotensin II (Ang II) (15,16). Peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α) is an important regulator of energy metabolism and mitochondrial function. Adipocytes can secrete a variety of cytokines, and when the mother is obese, these obesity cytokines also affect endothelial cell function. It has been shown that PGC-1α has the function of upregulating VEGFA and other angiogenic factors, thereby enhancing angiogenesis and promoting the formation of new blood vessels (17). Sirtuin 1 (SIRT1) is a functional regulator of PGC-1α, a metabolic gene transcription program that induces mitochondrial fatty acid oxidation. One study has demonstrated that SIRT1 can deacetylate PGC-1α, increasing its activation (18). It is a homolog of the silent information regulator 2 (SIR2) (19). Recent experimental analyses have demonstrated that SIRT1 plays a crucial role not only in many cellular pathways, including those involved in cellular survival, apoptosis, oxidative stress, and energy metabolism (20), but also in the regulation of angiogenesis. When oxidative stress occurs in pregnant women, reactive oxygen species (ROS) increase greatly, causing vascular endothelial cell damage in pregnant women. At the same time, the content of inflammatory factors such as tumor necrosis factor-α (TNF-α) in pregnant women is increases, which damages the physiological function of placenta, leading to pathological pregnancy, eclampsia and other adverse symptoms. Previous studies have shown that SIRT1 deletion in human umbilical vein endothelial cells (HUVECs) leads to hyperactivation of the Notch signaling pathway, blocking of the growth and budding of HUVECs, and reduced vascular density and vascular branching pattern (21). In zebrafish and mice, the research has found that SIRT1 knockdown increases the acetylation of FOXO1, resulting in vasculature formation defects (22).
Altogether, SIRT1 and PGC-1α play key roles in the regulation of angiogenesis. However, the effect of SIRT1/PGC-1α on HUVECs during placental angiogenesis in a high fat environment remains unclear. In consequence, a rat pre-pregnancy obesity model was established, with which to investigate the effect of maternal obesity on SIRT1/PGC-1α pathway expression levels and angiogenesis in the placenta. There are few studies on the mechanism of SIRT1/PGC-1α in prepregnancy obesity. To explore whether SIRT1/PGC-1α is involved in the physiological process of prepregnancy obesity can provide more treatment methods for the treatment of prepregnancy obesity. The HUVECs were cultured in medium containing palmitate acid to mimic the high fat environment of placentation (23). An inhibitor and an activator of the SIRT1/PGC-1α pathway were used to determine whether the pathway could influence the proliferation, migration, and tubule formation of HUVECs in in vitro experiments. We aimed to explore whether maternal obesity inhibits placental angiogenesis through down-regulation of SIRT1/PGC-1α signaling pathway. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1221/rc).
Methods
Materials
Palmitic acid (PA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies against SIRT1, PGC-1α, and VEGFA were purchased from Abcam (Cambridge, UK). We purchased SRT1720, a SIRT1 activator, and EX-527, a SIRT1 inhibitor, from Sigma-Aldrich, dissolved in dimethyl sulfoxide (DMSO) at a concentration of 5 mg/mL, and stored at −80 ℃.
Animal models
All animals were used only for medical research. Sprague-Dawley (SD) rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). We used 6-week-old female rats were used for the high fat diet (HFD) animal model. All female SD rats were housed in a standard environment with a 12 h/12 h light/dark cycle, with free access to water. A total of 20 healthy SD rats were randomly divided into 2 groups with 10 rats in each group. The SD rats were fed with normal diets or HFD (comprising 60% Kcal from fat. After 6 weeks, the females were mated. Embryonic day 0.5 (E0.5) was defined by the presence of a vaginal plug. The normal group was fed the standard diet, while the HFD group was fed the HFD. All pregnant rats were sacrificed on gestational day (GD) 18.5. Placentas, maternal serum samples, and fetuses were obtained after cesarean section. Part of the specimens were fixed in stationary liquid and embedded with paraffin for tissue staining. The other part was used for the extraction of RNA and protein. Animal experiments were approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJH-202104014), in compliance with Tongji Hospital guidelines for the care and use of animals. Measures were taken to minimize the use of animals and their suffering.
Immunohistochemistry
The placental tissues were extracted and fixed in 4% paraformaldehyde solution for 24 h, embedded in fresh paraffin, then cut into 5 µm sections. After the tissue sections were dewaxed with xylene and ethanol, ethylenediamine tetraacetic acid (EDTA) antigen retrieval buffer was used for antigen retrieval. Specific staining was reduced by incubation with 3% H2O2. Subsequently, the tissue sections were washed with phosphate-buffered saline (PBS) 3 times, and the nonspecific binding sites were blocked by incubation with 10% goat serum. Sections were incubated overnight with primary antibody (Abcam, Cambridge, MA, USA) CD31 (1:50) at 4 ℃, washed with PBS, and incubated with secondary antibody IgG (1:200) at 37 ℃ for 30 min. After washing with PBS, diaminobenzidine was used for 3–5 min to develop color, and the tissue was washed with water to stop the color reaction. The slices were subsequently stained with hematoxylin. Images were taken under a microscope.
Hematoxylin and eosin staining
Tissue sections, 5 μm thick, cut from paraffin blocks, were fixed on poly-L-lysine-coated slides. The tissue sections were dewaxed by xylene and gradient ethanol and stained with hematoxylin for 3 min (24). They were then differentiated with 1% ethyl alcohol hydrochloride for 30 s, dyed with 0.5% eosin for 3 min, then dehydrated and made transparent with gradient ethanol and xylene successively. After drying in a fume hood, the sheet was sealed with neutral gum. The histopathological morphological changes were observed under a microscope.
Cell culture
We obtained HUVECs from the China Center for Type Culture Collection (CCTCC; Hubei, China). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) at 37 ℃, under 5% carbon dioxide. For the experiments, the cells were divided into 4 groups: a control group (complete culture medium only), a PA (palmitic acid; 0.4 mM) group, a SRT1720 (activator of SIRT1; 3 µM) + PA group, and an EX-527 (inhibitor of SIRT1; 5 µM) + PA group. Sorted cells were cultured for 24 h under the conditions described above for the next experiments.
Quantitative reverse transcription polymerase chain reaction
Total RNA was extracted from rat placental tissue and HUVECs using TRIzol reagent. The RNA samples were detected by gel electrophoresis [electrophoresis condition: 1.2% gel, 0.5×Tris-borate-EDTA (TBE) electrophoresis buffer] and optical density (OD)260 nm/OD280 nm values. For each sample, the isolated RNA was reverse-transcribed into complementary DNA (cDNA) using the ReverTra Ace® qPCR RT Master Mix with gDNA remover kit (Toyobo Co., Ltd., Osaka, Japan). Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed on a LightCycler 480 System (Roche Diagnostics, Basel, Switzerland) using SYBR Premix Ex Taq (Takara Bio Inc., Shiga, Japan). The primers used were as follows: SIRT1 (F: 5'-GCTCGCCTT GCTGTGGACTTC-3', R: 5'-GTGACACAG AGATGGCTGGAACTG-3'); PGC-1α (F: 5'-CGGTGGATGAAGACGGATTGCC-3', R: 5'-ATTGTAGCTGAGCTGAGTGTTGGC-3'); VEGFA (F: 5'-GCTGTCTTGGGTGCATT GG-3, R: 5'-GCAGCCTGGGACCACTTG-3'); and GAPDH (F: 5'-ACGGCAAGTTCAACGGCACAG-3', R: 5'-CAGCATACTCAGCACCAGCATCAC-3'). The 5.0 primers were designed and synthesized by Tsingke Biological Technology (Wuhan) Co., Ltd. (Hubei, China). Relative quantification was carried out using the 2−ΔΔCt method, and normalized to the amount of GAPDH. All samples were performed in at least triplicate.
Western blotting
Placental tissue samples and HUVECs were lysed using radioimmunoprecipitation assay (RIPA) lysis buffer containing protease and phosphatase inhibitors (Boster Bio, Pleasanton, CA, USA), and total protein lysates were extracted. The protein concentration was determined using the bicinchoninic acid (BCA) method (Pierce, Thermo Fisher Scientific, Waltham, MA, USA). The proteins were separated using 10% polyacrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was sealed with 5% skim milk at room temperature for 2 h, diluted primary antibody was added, and the membrane was incubated at 4 ℃ overnight. Horseradish peroxidase (HRP)-labeled secondary antibody IgG (Abcam, USA) was incubated for 1 h at room temperature. After washing with tris-buffered saline with Tween 20 (TBST), the membrane was color-developed using an ECL luminescent solution (Pierce, Thermo Fisher Scientific, USA). Protein strips were recorded and processed using a Bio-Rad exposure machine (Bio-Rad, Hercules, CA, USA). The relative protein expression was calculated as the ratio of target band to β-actin gray value. The other reagents used were: Primary antibody (Abcam); SIRT1 (ab110304, dilution ratio 1:1,000); PGC-1α (ab106814, dilution ratio 1:1,000); VEGF-A (ab51745, dilution ratio 1:1,000); and β-actin (ab8226, dilution ratio 1:1,000).
Cell proliferation assays
Cell proliferation was detected using cell counting kit-8 (CCK-8) assays. Approximately 5×103 HUVECs per well induced with PA, SRT1720, EX-527, and controls were plated in 96-well plates and placed in an incubator at 37 ℃ for 24 h. Then, 10 µL of CCK-8 reagent was added to each well, and the plates were incubated at 37 ℃ for another 2 h. Cell proliferation was measured by detecting the OD at 450 nm using a microplate reader.
In vitro wound assays
The HUVECs were inoculated into 6-well plates as a cell concentration of 3×105 cells per well. When the degree of cell fusion reached 90%, they were used for experiments. The serum was removed from the medium and the HUVECs were cultured for 4 h. A cultured monolayer of HUVECs was scratched using a 200 µL pipette, and then washed with PBS 3 times. A single layer of cells in the medium showed several gaps without cells. These cells were placed in 4 groups: Control group (complete culture medium only), PA (palmitic acid; 0.4 mM) group, SRT1720 (activator of SIRT1; 3 µM) + PA group, EX-527 (inhibitor of SIRT1; 5 µM) + PA group. We observed and photographed 3 randomly selected gaps under a microscope. After incubation at 37 ℃ for 24 h, images were recorded, and the cell migration rate was evaluated using Image J software (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/).
Tubule formation experiments
Matrigel was defrosted at 4 ℃, and added to the 96-well cell culture plates using precooled pipette tips. The Matrigel was placed in a refrigerator at 4 ℃ for 30 min, and then in an incubator at 37 ℃ for 30 min. Approximately 1×105 HUVECs per well induced with PA, SRT1720, and EX-527, and controls, were seeded into each of the Matrigel-coated 96-well plates in triplicate and incubated at 37 ℃. After 24 h of incubation, the tube areas were observed under a microscope and photographed. The total tube length and the number of nodes were calculated using Image J.
Statistical analysis
Results were analyzed using the software SPSS 23.0 (IBM Corp., Armonk, NY, USA). Significant differences relative to the controls were determined using independent-sample t-tests. For multiple group comparisons, one-way analysis of variance (ANOVA) was used to determine the significant differences. All results were presented as mean ± standard, and P values <0.05 (2-tailed) were considered statistically significant. Each experiment was repeated 3 times.
Results
HFD caused maternal obesity and decreased the ratio of fetal to placental weight
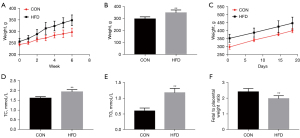
The impacts of HFD before conception on weight gain and the serum lipid levels of dams were analyzed. There were significant differences in the body weights of dams in the different study groups at the initiation of the diets. By the time of mating, the rats in the HFD groups were significantly heavier than the controls (Figure 1A-1C). Maternal HFD increased maternal fasting triglyceride and fasting total cholesterol (Figure 1D,1E). To investigate the effects of pregnancy before HFD feeding on placental efficiency, we examined the ratio of fetal to placental weight. The lower fetal to placental weight ratio in HFD group indicated that nutrient transport efficiency of placenta was lower than average (Figure 1F). It could be seen that a HFD weakens the efficiency of the placenta. Overall, the HFD had a negative impact on both placental vascular production and function.
Exposure to maternal HFD impaired placental angiogenesis and decreased the expression of SIRT1/PGC-1α
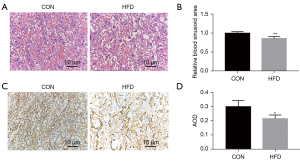
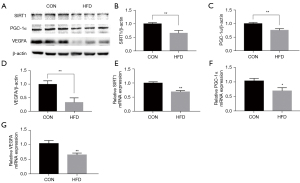
To evaluate the effect of maternal HFD feeding on the vascular density and structure of the placenta, the histology of the placentas was examined using hematoxylin-eosin (HE) stains. The placental trophoblasts were sparse and disordered, the capillaries were significantly reduced and unevenly distributed, and the number of red cells was reduced in HFD groups compared with controls (Figure 2A,2B). As a vascular endothelial cell marker, CD31 is used to evaluate blood vessel generation. The CD31 immunohistochemistry (IHC) showed a strongly positive immunoreaction in villous endothelial cells, and the expression level of CD31, as represented by average optical density (AOD), was reduced in the HFD groups (Figure 2C,2D). We detected SIRT1/PGC-1α/VEGFA in the placental tissues of pregnant dams. We collected placental tissues from the control group (CON) and HFD groups, and analyzed the expression of SIRT1/PGC-1α and VEGFA. The SIRT1/PGC-1α and VEGFA messenger RNA (mRNA) and relative protein levels in the placentas of the HFD group were lower than those in the CON group (Figure 3A-3G). These results suggest that maternal obesity impaired placental angiogenesis and downregulated the expression levels of SIRT1/PGC-1α and VEGFA at both the mRNA and protein levels.
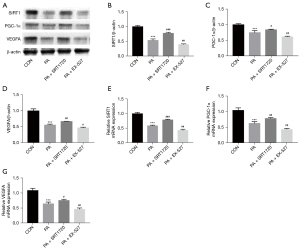
SRT1720 activated the SIRT1/PGC-1α pathway
The SIRT1 is known to be a NAD+-dependent deacetylase SIRT1, which regulates PGC-1α activity through deacetylation. We investigated the expression of SIRT1/PGC-1α and VEGFA (angiogenic factor) in HUVECs under high fat conditions. Protein levels of SIRT1, PGC-1α and VEGFA were decreased in HUVECs after PA treatment (Figure 4A-4D). Correspondingly, mRNA levels of SIRT1, PGC-1α and VEGFA were significantly downregulated (Figure 4E-4G). These data suggest that high fat stress can inhibit the SIRT1/PGC-1α pathway in HUVECs. After treatment with the SIRT1 activator SRT1720, the expression of SIRT1, PGC-1α, and VEGFA in PA-induced HUVECs was significantly increased. We also investigated the effect of the SIRT1 inhibitor EX-527 on the expression of SIRT1/PGC-1α and VEGFA in PA-induced HUVECs. The results showed that the relative protein and mRNA expression of SIRT1, PGC-1α, and VEGFA was further inhibited. Collectively, these results suggest that SRT1720 reduced the inhibition of the SIRT1/PGC-1α and VEGFA expression induced by high fat stress, while EX-527 aggravated the SIRT1/PGC-1α and VEGFA inhibition.
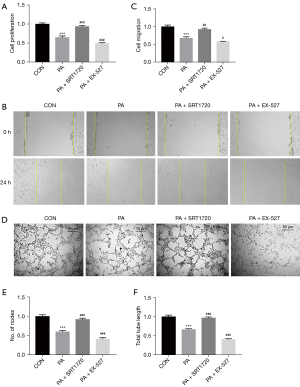
SIRT1 activation promoted HUVECs proliferation, migration, and angiogenesis in vitro
In order to investigate the effect of PA on endothelial cell function, and determine whether SIRT1 can ameliorate the damage caused by PA to endothelial cell function, we examined the migration, proliferation, and tube-forming abilities of the HUVECs in each group. Since endothelial cell proliferation is also a key factor affecting angiogenesis, CCK-8 assays were performed. The CCK-8 assays showed that cell proliferation was inhibited in a high-fat environment. Compared with cells under high-fat conditions, SRT1720 treatment restored the inhibition of cell proliferation, while EX-527 treatment further inhibited cell proliferation (Figure 5A). The results of cell scratch experiments showed that the migration ability of HUVECs cells was weakened under high-fat conditions, and the difference from the control group was significant. Therefore, SRT1720 treatment of HUVECs can relieve the inhibition of migration ability of HUVECs in a high-fat environment. In contrast, treatment with EX-527 increased the inhibition of migration (Figure 5B,5C). Compatible with the results of the cell migration and proliferation experiments, the tube formation experiment showed that the number of nodes and the tube length of HUVECs were decreased after 24 h of culture in high-fat medium, compared with the control group. Treatment with SRT1720 increased the number of nodes and the tube length of HUVECs induced with PA, compared with the PA group. In contrast, treatment with EX-527 further reduced the number of nodes and the total length of the tube compared with the PA group (Figure 5D-5F). Taken together, these results suggest that high-fat conditions reduce the angiogenic capacity of HUVECs by restraining proliferation, migration, and tubule formation. However, activation of the SIRT1/PGC-1α pathway attenuated the inhibitory effect of the angiogenic capacity of HUVECs caused by high-fat conditions.
Discussion
Blood flow in the placenta vascular network is one of the main determinants of normal placental function (25). The formation of the placental vasculature, a complex physiological process, involves 2 consecutive processes of vasculogenesis and angiogenesis (26). The process of de novo formation of blood vessels from endothelial progenitor cells or angioblasts is called vasculogenesis (27). Placental angiogenesis, which follows vasculogenesis, includes branching angiogenesis in the first trimester of pregnancy, and non-branching angiogenesis in the third trimester of pregnancy (28). Angiogenesis is a highly regulated process, involving the proliferation, migration, and tube formation of vascular endothelial cells (29). There is a growing body of evidence which indicates that an abnormal vascular network in the placenta leads to multiple adverse pregnancy outcomes, including preeclampsia, intrauterine growth restriction (IUGR), recurrent spontaneous miscarriage, and fetal death (30-33). It is important to understand the mechanism of regulation of angiogenesis in the placenta under conditions of gestational obesity.
As an energy sensor, SIRT1 plays an essential role in the regulation of metabolism and is a key regulator of angiogenesis (34-36). Moreover, VEGF and its 2 receptors, VEGFR-1 and VEGFR-2, are expressed in the endothelium of blood vessels, and are essential to vascular development and the regulation of vascular permeability (37). In the present study, we demonstrated that maternal obesity impaired placental angiogenesis and reduced the expression of SIRT1, PGC-1α, and VEGFA, compared to the controls. In in vitro experiments, SIRT1/PGC-1α was found to play an essential role in promoting the proliferation, migration, and tube formation capacity of HUVECs. These results indicate that the SIRT1/PGC-1α signaling pathway plays an important role in the regulation of placental angiogenesis under high-fat conditions.
In our experiment, HFD dams had higher plasma triglyceride levels in late gestation, consistent with previous studies using a 60% HFD diet during gestation (13,38,39). The placenta is an important organ at the maternal-fetal interface, which supports the normal growth and development of the fetus, and the fetal/placental weight ratio is a useful measure of placental efficiency (40). During pregnancy, the level of desaturase FADS2 decreased and fatty acid metabolism was blocked in high-fat diet female rats, which led to the up-regulation of placental inflammatory factors and the structural and functional disorders of placenta. In this study, we investigated the impact of an HFD diet before conception and throughout pregnancy on the placental efficiency. The ratio of fetal weight to placental weight was reduced in the HFD group. Research has suggested that hyperlipidemia leads to poor placental vasculature, leading to reduced oxygenation and low neonatal survival (41). Serving as a marker of vascular endothelial cells, CD31 IHC was conducted to evaluate angiogenesis. It was revealed that blood vessel density was reduced in HFD dams. A recent study showed that SIRT1 overexpression upregulates VEGF expression, and may exert a therapeutic role, attenuating inflammation by regulating angiogenic imbalance in PE rats (42). In the present study, as expected, the number of CD31-positive cells was reduced in HFD dams. We measured the total levels of SIRT1, PGC-1α, and VEGFA in placental tissues, and found that SIRT1, PGC-1α, and VEGFA levels were decreased in HFD dams. Our data suggest that HFD feeding before and during pregnancy induces maternal obesity in rats and inhibits placental angiogenesis, thereby reducing placental efficiency.
The HUVECs have been extensively used as a model cell type in a number of studies of placental angiogenesis. We used HUVECs to further investigate the roles of SIRT1/PGC-1α in placental angiogenesis. In this study, PA was used to simulate the high-fat microenvironment of HUVECs and establish the high-fat model. Our results showed that the proliferation and migration of HUVECs were downregulated, and the angiogenesis of HUVECs was weakened under the high-fat conditions in vitro. The decreased expression of SIRT1/PGC-1α and VEGFA was responsible for the decreased proliferation and migration of HUVECs, and the inhibition of angiogenesis. Other studies have also shown that reduced SIRT1 activity leads to age-related dysfunction and decreased angiogenesis of HUVECs (22,34). Therefore, obesity can reduce the expression of SIRT1/PGC-1α and VEGFA, and may lead to the dysfunction of HUVECs and the inhibition of angiogenesis in placentas.
The small-molecule activator, SRT1720, can activate SIRT1, which enhances the expression and activity of SIRT1 without interfering with other sirtuin proteins. Conversely, EX-527, a small-molecule inhibitor, exhibits an inhibitory effect on SIRT1 expression and activity (43). In the present study, we found the reduction in the proliferation, migration, and tube formation capacity of HUVECs under high-fat conditions was promoted by SRT1720, producing increased levels of SIRT1/PGC-1α and VEGFA. This promotion could be reversed by EX-527. The SIRT1/PGC-1α signaling pathway alters the expression of downstream genes and dysregulates the angiogenic function of HUVECs. Thus, we also found that VEGFA expression changes are synchronistic with those of SIRT1/PGC-1α. SIRT1 may promote the expression of VEGFA by activating PGC-1α, and thus promote the formation of placental blood vessels. Together, these results supported the hypothesis that activation of the SIRT1/PGC-1α signaling pathway could effectively improve angiogenesis of HUVECs under high-fat conditions, and these changes probably occur via the expression of VEGFA. In this study, the contents of triglyceride and cholesterol in obese rats during pregnancy increased, and the fat metabolism pathway of rats was also affected. The effect of SIRT1/PGC-1α in pregnant rats may be related to the fat metabolism pathway.
In conclusion, these data suggest that maternal obesity inhibits placental angiogenesis by down-regulating the SIRT1/PGC-1α signaling pathway, and that SIRT1/PGC-1α activation may prevent the impaired placental angiogenesis in a high-fat state by increasing the expression of VEGFA. However, there were limitations to the present study. Another important transcription factor in energy metabolism is PGC-1α. Whether the downstream effector gene of PGC-1α is changed by maternal obesity needs further study. However, we believe that our results will not be reversed by this effect and that our results will retain their reliability.
Acknowledgments
The authors extend their thanks to all those who contributed to this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1221/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1221/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1221/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animals experiments were approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJH-202104014), in compliance with Tongji Hospital guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288-98. [Crossref] [PubMed]
- van Dijk SJ, Tellam RL, Morrison JL, et al. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin Epigenetics 2015;7:66. [Crossref] [PubMed]
- Bray GA, Kim KK, Wilding JPH, et al. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017;18:715-23. [Crossref] [PubMed]
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017;356:j1. [Crossref] [PubMed]
- Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol 2015;30:1141-52. [Crossref] [PubMed]
- Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr 2015;101:302-9. [Crossref] [PubMed]
- Desai M, Ross MG. Fetal programming of adipose tissue: effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin Reprod Med 2011;29:237-45. [Crossref] [PubMed]
- Dimasuay KG, Boeuf P, Powell TL, et al. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front Physiol 2016;7:12. [Crossref] [PubMed]
- Song T, Lu J, Deng Z, et al. Maternal obesity aggravates the abnormality of porcine placenta by increasing N6-methyladenosine. Int J Obes (Lond) 2018;42:1812-20. [Crossref] [PubMed]
- Zhou Y, Xu T, Cai A, et al. Excessive backfat of sows at 109 d of gestation induces lipotoxic placental environment and is associated with declining reproductive performance. J Anim Sci 2018;96:250-7. [Crossref] [PubMed]
- Bi WG, Nuyt AM, Weiler H, et al. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr 2018;172:635-45. [Crossref] [PubMed]
- Song L, Sun B, Boersma GJ, et al. Prenatal high-fat diet alters placental morphology, nutrient transporter expression, and mtorc1 signaling in rat. Obesity (Silver Spring) 2017;25:909-19. [Crossref] [PubMed]
- Stuart TJ, O'Neill K, Condon D, et al. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol Reprod 2018;98:795-809. [Crossref] [PubMed]
- Saben J, Lindsey F, Zhong Y, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014;35:171-7. [Crossref] [PubMed]
- Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation 2014;21:15-25. [Crossref] [PubMed]
- Azevedo Portilho N, Pelajo-Machado M. Mechanism of hematopoiesis and vasculogenesis in mouse placenta. Placenta 2018;69:140-5. [Crossref] [PubMed]
- Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008;451:1008-12. [Crossref] [PubMed]
- Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113-8. [Crossref] [PubMed]
- Wang Y, Zhao X, Shi D, et al. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis Sci 2013;54:3806-14. [Crossref] [PubMed]
- Strycharz J, Rygielska Z, Swiderska E, et al. SIRT1 as a Therapeutic Target in Diabetic Complications. Curr Med Chem 2018;25:1002-35. [Crossref] [PubMed]
- Guarani V, Deflorian G, Franco CA, et al. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature 2011;473:234-8. [Crossref] [PubMed]
- Potente M, Ghaeni L, Baldessari D, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 2007;21:2644-58. [Crossref] [PubMed]
- Kakigano A, Tomimatsu T, Mimura K, et al. Drug Repositioning for Preeclampsia Therapeutics by In Vitro Screening: Phosphodiesterase-5 Inhibitor Vardenafil Restores Endothelial Dysfunction via Induction of Placental Growth Factor. Reprod Sci 2015;22:1272-80. [Crossref] [PubMed]
- Song YP, Chen YH, Gao L, et al. Differential effects of high-fat diets before pregnancy and/or during pregnancy on fetal growth development. Life Sci 2018;212:241-50. [Crossref] [PubMed]
- Poston L. The control of blood flow to the placenta. Exp Physiol 1997;82:377-87. [Crossref] [PubMed]
- Kalka C, Asahara T, Krone W, et al. Angiogenese und Vaskulogenese. Therapeutische Strategien zur Stimulation der postnatalen Neovaskularisation. Herz 2000;25:611-622. [Crossref] [PubMed]
- Ayala-Domínguez L, Olmedo-Nieva L, Muñoz-Bello JO, et al. Mechanisms of Vasculogenic Mimicry in Ovarian Cancer. Front Oncol 2019;9:998. [Crossref] [PubMed]
- Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction 2009;138:895-902. [Crossref] [PubMed]
- Nie D, Tang K, Diglio C, et al. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood 2000;95:2304-11. [Crossref] [PubMed]
- Mandò C, De Palma C, Stampalija T, et al. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am J Physiol Endocrinol Metab 2014;306:E404-13. [Crossref] [PubMed]
- Figueras F, Gratacos E. Stage-based approach to the management of fetal growth restriction. Prenat Diagn 2014;34:655-9. [Crossref] [PubMed]
- Girardi G, Yarilin D, Thurman JM, et al. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006;203:2165-75. [Crossref] [PubMed]
- Gasperowicz M, Otto F. The notch signalling pathway in the development of the mouse placenta. Placenta 2008;29:651-9. [Crossref] [PubMed]
- Das A, Huang GX, Bonkowski MS, et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018;173:74-89.e20. [Crossref] [PubMed]
- Qiang L, Sample A, Liu H, et al. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci Rep 2017;7:14110. [Crossref] [PubMed]
- Lin Y, Li L, Liu J, et al. SIRT1 Deletion Impairs Retinal Endothelial Cell Migration Through Downregulation of VEGF-A/VEGFR-2 and MMP14. Invest Ophthalmol Vis Sci 2018;59:5431-40. [Crossref] [PubMed]
- Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem 2007;109:257-65. [Crossref] [PubMed]
- Xie Q, Gu X, Chen J, et al. Soyasaponins Reduce Inflammation and Improve Serum Lipid Profiles and Glucose Homeostasis in High Fat Diet-Induced Obese Mice. Mol Nutr Food Res 2018;62:e1800205. [Crossref] [PubMed]
- Wentzel P, Eriksson UJ, Herrera E. High-fat diet in pregnant rats and adverse fetal outcome. Ups J Med Sci 2019;124:125-34. [Crossref] [PubMed]
- Fowden AL, Sferruzzi-Perri AN, Coan PM, et al. Placental efficiency and adaptation: endocrine regulation. J Physiol 2009;587:3459-72. [Crossref] [PubMed]
- Hayes EK, Lechowicz A, Petrik JJ, et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS One 2012;7:e33370. [Crossref] [PubMed]
- Huang Y, Zheng XD, Li H. Protective role of SIRT1-mediated Sonic Hedgehog signaling pathway in the preeclampsia rat models. J Assist Reprod Genet 2021;38:1843-51. [Crossref] [PubMed]
- Bai X, Yao L, Ma X, et al. Small Molecules as SIRT Modulators. Mini Rev Med Chem 2018;18:1151-7. [Crossref] [PubMed]
(English Language Editor: J. Jones)


