
DAPK2 activates NF-κB through autophagy-dependent degradation of I-κBα during thyroid cancer development and progression
Introduction
Thyroid cancer (TC) is the most common endocrine malignancy, and its incidence has risen worldwide over the past few decades (1). Although most thyroid carcinomas have well-differentiated papillary histology and good prognosis, patients with more aggressive tumor subtypes, such as anaplastic thyroid cancer (ATC), already show local and distant metastasis at the time of diagnosis, and are usually resistant to standard chemotherapy. Consequently, understanding the molecular mechanisms of these carcinomas is a key research imperative in TC therapeutics.
Autophagy, a process of self-digestion, is essential for the maintenance of cellular homeostasis (2). Autophagy has been shown to be involved in several steps of TC pathogenesis, including tumor development and progression, as well as resistance to chemo- and radiotherapy (3). Intervention of this process may therefore present a novel approach in the management of TC therapy.
Death-associated protein kinase 2 (DAPK2) belongs to a family of Ca2+/calmodulin-regulated serine/threonine kinases, which regulate various cellular activities, including cell death, cytoskeletal dynamics, and immune functions. Recent studies suggest that DAPK2 is involved in several steps of autophagy. DAPK2 functions as a tumor suppressor in several cancers like hepatocellular carcinoma (HCC) and acute myeloid leukemia (AML). However, the role of DAPK2 in TC development has not yet been investigated.
Much progress has been made in understanding the molecular mechanisms of TC in recent years (4). Emerging evidence has shown that deregulated nuclear factor-κB (NF-κB) activation is associated with thyroid tumor initiation and progression, especially in ATC in which constitutive NF-κB activity has been found (5-8). NF-κB promotes TC progression by controlling proliferative and antiapoptotic signaling pathways in TC cells (9,10).
Here, we show that DAPK2 is upregulated in human TC samples and plays a critical role in TC pathogenesis, being required for tumor growth and for the resistance to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, through selective autophagy mediated inhibitory-κBα (I-κBα) degradation, which correlates with strong of NF-κB activity. This makes DAPK2 a target for the development of novel therapeutic strategies for TC.
We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-2062).
Methods
Cell lines
Human anaplastic TC cell line TTA1 and human embryonic kidney cell line HEK293T were purchased from the American Type Culture Collection (ATCC) and grown in high glucose Dulbecco’s Modified Eagle Medium (DMEM) medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 µg/mL streptomycin (GIBCO, USA).
RNA interference of DAPK2
The following oligos were used for the construction of short hairpin (sh) RNAs against DAPK2: shDAPK2-1: 5'-TGCTGTTGACAGTGAGCGACCGGAATTTGTTGCTCCAGAATAGTGAAGCCACAGATGTATTCTGGAGCAACAAATTCCGGCTGCCTACTGCCTCGGA-3', shDAPK2-2: 5'-TGCTGTTGACAGTGAGCGCCAGAGAGAGACCATATCCAAATAGTGAAGCCACAGATGTATTTGGATATGGTCTCTCTCTGTTGCCTACTGCCTCGGA-3'. The oligos were amplified using the primer pair 5'-CAGAAGGCTCGAGAAGGTATATTGCTGTTGACAGTGAGCG-3' and 5'-CTAAAGTAGCCCCTTGAATTCCGAGGCAGTAGGCA-3' to introduce the miR-30 sequence, and XhoI and EcoRI restriction sites, and then were inserted into pMLP vector (Transomics, Shanghai, China). Recombinant MLP vector containing shDAPK2-1, shDAPK2-2, or non-targeting control combined with pCL-10A1 retrovirus packaging vector were transfected into HEK293T cells using Lipofectamine 2000 (Life Technologies, USA) according to manufacturer’s instructions. After 48-hour incubation, lentiviruses were collected from culture supernatant. TTA1 cells were transfected with retroviruses in the presence of 10 µg/mL of polybrene and selected by 1 µg/mL of puromycin. The efficiency of RNA interference was determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot.
Ectopic expression of DAPK2
Human DAPK2 cDNA was cloned into the pCDH vector. PCDH-DAPK2 or vector control with pSPAX2 and pMD2.G lentivirus packaging vectors were transfected into HEK293T cells using Lipofectamine 2000 (Life Technologies). After 48-hour incubation, lentiviruses were collected from culture supernatant. TTA1 cells were infected with the lentiviruses in the presence of 10 µg/mL of polybrene. The infected cells were screened by 1 µg/mL of puromycin. DAPK2 expression levels were assessed by both qRT-PCR and western blot.
qRT-PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, USA). Complement DNA (cDNA) was prepared using Reverse Transcriptase kit (Takara, China). RNA levels were determined using SYBR Green Real-time PCR master mix (Takara) on a 7900 HT qRT-PCR machine (Applied Biosystems, USA). The following pair of primers of DAPK2 were used for amplification: 5'-AGGCGTCATCACCTACATCC-3' and 5'-GAGCCTCTTGGATTGTGAGC-3'; beta Actin (ACTB) gene was used as an internal control. The primers of ACTB were 5'-CACCATTGGCAATGAGCGGTTC-3' and 5'-AGGTCTTTGCGGATGTCCACGT-3'. Relative messenger RNA (mRNA) expression levels were calculated as the ratio of DAPK2 to ACTB using the ΔΔCt method.
Western blot analysis
Cells were lysed with radio immunoprecipitation assay buffer (RIPA, Beyotime Biotech, China) supplemented with 1% protease inhibitor and 1 mM phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich, USA). For detecting phosphorylated proteins, phosphatase inhibitors (1 mM NaF, 1 mM sodium vanadate, 25 mM βGPO4) were also added. Protein concentration was measured using a bicinchoninic acid (BCA) protein assay kit (Tiangen, China). Equal amounts of protein were resolved onto SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% (w/v) nonfat milk, incubated with primary and horseradish peroxidase (HRP)-conjugated secondary antibodies, and then detected with an enhanced chemiluminescence (ECL) kit (Thermo Fisher Scientific, USA). The following antibodies were used to probe the corresponding proteins: DAPK2 (Abcam, USA), microtubule-associated protein 1A/1B-light chain 3 (LC3) B (Cell Signaling Technology, USA), β-actin (CST, USA), and HRP-conjugated goat anti-rabbit immunoglobin G (IgG; Cell Signaling Technology, USA).
Cell proliferation assay
TTA1 cells were seeded into 12-well plates at a concentration of 1×104 cells/well. Cell numbers were recorded using a cell counter (Bio-Rad, USA) for 7 consecutive days. Growth curves of the cells were plotted as the number of cells versus days.
Apoptosis assay
TTA1 cells were seeded into 24-well plates (1×105 cells/well). Cells were treated with TRAIL (100 ng/mL) for 24 hours, harvested, washed with phosphate-buffered saline (PBS) and then stained with Annexin V and 1 µL of propidium iodide (PI; eBioscience, USA) according to the manufacturer’s instructions. Cell apoptosis was detected and analyzed by Accuri C6 (BD Biosciences, USA).
Soft agar colony-formation assay
Subsequently, 1×103 of TTA1 cells was resuspended in DMEM medium containing 10% FBS and 0.3% low-melting agarose (Sangon Biotech, China). Cells were plated onto 60-mm culture dishes with the solidified bottom layer in DMEM medium containing 10% FBS and 0.5% low-melting agarose. On day 14, the colonies were fixed with methanol, stained with 0.05% crystal violet, and imaged.
Tumor growth in nude mice
Six-week-old male nude mice were purchased from Shanghai Laboratory Animal Center (SLAC) Laboratory Animal Company (Shanghai, China). Mice were housed in a specific pathogen-free (SPF) room at 24±1 °C and with a relative humidity of 55%±5%. Control, shDAPK2, or DAPK2 OE TTA1 cells were subcutaneously injected (1×106/mouse) into the back of nude mice (6 mice/group). Tumor growth was monitored for 6 weeks. Tumors were weighted and imaged after surgical removal. Animal experiments were performed under a project license (No. SH9H-2020-T346-1) granted by institutional ethics committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, in compliance with Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine institutional guidelines for the care and use of animals.
Immunofluorescence staining and confocal laser microscopy
The cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and then blocked with 1% bovine serum albumin (BSA). The cells were then incubated with primary antibodies against LC3B (Abcam, USA) overnight at 4 °C. After washing with PBS, the cells were incubated with FITC-conjugated secondary antibodies (Abcam, USA) for 0.5 hours, stained with DAPI for 2 minutes, and then observed by confocal fluorescence microscopy.
NF-κB luciferase reporter assay
TTA1 cells were transfected with firefly luciferase gene reporter plasmid encoding pNF-κB Luc using Lipofectamine 2000 (Life Technologies). Transfection efficiency was normalized by cotransfection with the Renilla luciferase plasmid. The cells were lysed 48 hours after transfection, and luciferase activity was measured following the manufacturer’s instructions (Promega, USA). NF-κB transcriptional activity was analyzed as the ratio of firefly luciferase activity to Renilla luciferase activity.
Human TC specimens
Three pairs of TC and adjacent noncancerous tissue samples of TC patients were obtained from the Shanghai Ninth People’s Hospital for RNA sequencing (RNA-seq) and qRT-PCR analysis. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine institutional ethics committee (No. SH9H-2020-T346-1) and informed consent was taken from all the patients.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical differences were determined by two-tailed Student’s t-test using GraphPad Prism 5.0 software. A P value <0.05 was considered statistically significant.
Results
DAPK2 was upregulated in TC
To identify the novel functional genes involved in TC, we performed RNA-seq using an Illumina HiSeq Sequencer to select differentially expressed genes (Figure 1A). Among the upregulated genes, DAPK2 was picked due to its role in autophagy. We validated the DAPK2 mRNA expression level using qRT-PCR (Figure 1B). The relationship between DAPK2 and TC has not been previously reported. Therefore, we sought to investigate the role and the underling mechanism of DAPK2 in TC pathogenesis.
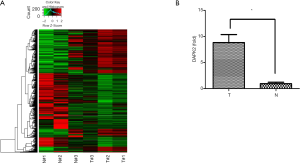
DAPK2 silencing inhibited the proliferation and tumorigenicity of TTA1 cells
To explore the role of DAPK2 in TC, we used shRNA to silence DAPK2 expression in TTA1 cells. The shRNAs effectively reduced the expression of DAPK2 in TTA1 cells compared to the non-targeting control, as indicated by western blot and qRT-PCR results (Figure 2A,B).
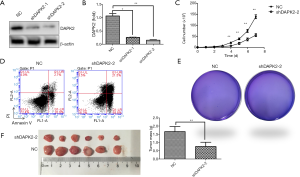
We first examined whether DAPK2 is involved in thyroid tumor growth control. An equal number of non-targeting control TTA1 cells and shDAPK2 cells were cultured for 7 days. As shown in Figure 2C, silencing of DAPK2 decreased the proliferation of TTA1 cells. It has been long held that carcinogenesis occurs as a result of the imbalance between proapoptotic or antiapoptotic signaling. TRAIL, a member of the TNF family has been shown to trigger apoptosis and selectively kill TC cells without damaging normal cells (11). However, some cancers, including TC, have developed resistance to TRAIL, limiting its anticancer potential. We thus investigated the effect of DAPK2 knockdown on sensitivity to TRAIL-induced cell death. Cells were challenged with TRAIL, and cell apoptosis were measured with flow cytometer, silencing DAPK rendered TTA1 cells more susceptible to the killing effect of TRAIL (Figure 2D). Next, we examined whether DAPK2 plays a role in anchorage-independent growth of TTA1 cells. As shown in Figure 2E, silencing of DAPK2 abolished TTA1 cell colony formation ability in a semisolid medium. To confirm these observations in vivo, an equal number of non-targeting control TTA1and shDAPK2 TTA1 cells were implanted s.c. into nude mice. As shown in Figure 2F, the weight and size of tumors developed from shDAPK2 TTA1 cells were significantly decreased. These results suggest that DAPK2 plays a critical role in the tumorigenicity of TTA1 cells.
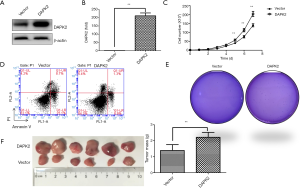
Ectopic expression of DAPK2 promoted proliferation and tumorigenicity of TTA1 cells
As knockdown of DAPK2 suppressed the proliferation and tumorigenicity of TTA1 cells, overexpression of DAPK2 might have an opposite effect. Therefore, DAPK2-expressing plasmid or vector control was transfected into TTA1 cells using the lentivirus system. As indicated by western blot and qRT-PCR analysis (Figure 3A,B), DAPK2 was highly expressed. In contrast with DAPK2 knockdown, overexpression of DAPK2 promoted proliferation of TTA1 cells (Figure 3C). Also, overexpression of DAPK2 led to resistance of TTA1 cells to TRAIL-induced apoptosis (Figure 3D). DAPK2 overexpression enhanced tumorigenicity of TTA1 cells both in vitro and in vivo, as indicated by soft agar colony-formation assay (Figure 3E) and xenograft tumor model (Figure 3F).
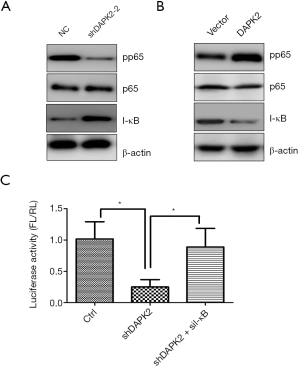
DAPK2 activated NF-κB through triggering degradation of I-κBα
As increased NF-κB activity has been shown to be associated with TC development and tumor progression, we aimed to determine whether enhanced NF-κB activation is involved in the tumorigenic effect of DAPK2. Western blot results showed that knockdown of DAPK2 decreased p65 phosphorylation, which correlated with increased I-κB protein level, while overexpression of TTA1 exhibited the opposite effect (Figure 4A,B). Dual luciferase reporter assay demonstrated that overexpression of DAPK2 significantly enhanced the transcriptional activity of NF-κB (Figure 4C), suggesting that DAPK2 positively regulates NF-κB signaling. We used small interfering (siRNA) to knockdown I-κBα in shDAPK2 TTA1 cells. Luciferase reporter assay showed that knockdown of I-κBα in shDAPK2 TTA1 cells restored the activity of NF-κB (Figure 4C), suggesting that DAPK2 activates NF-κB through promoting I-κBα degradation.
DAPK2 enhanced NF-κB activation through autophagy
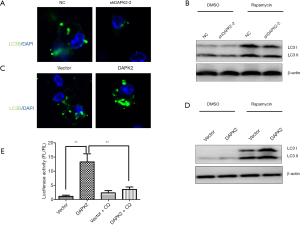
It was previously reported that DAPK2 is involved in selective autophagy (12). We therefore aimed to determine whether the effect of DAPK2 on I-κBα degradation and NF-κB activation occurs through autophagy. TTA1 cells were treated with 100 nM of rapamycin to induce autophagy. As expected, depletion of DAPK2 caused a decrease in the number of autophagosomes compared with the non-targeting control, as reflected by the appearance of LC3-FITC punctate staining (Figure 5A). Consistent with this, after rapamycin treatment, there was a reduction in the LC3II/LC3I ratio in shDAPK2 cells compared with the non-targeting control (Figure 5B). Conversely, overexpression of DAPK2 caused an increase in LC3 puncta and LC3II/LC3I ratio compared with vector control (Figure 5C,D). Furthermore, inhibition of autophagy by chloroquine diminished the effect of activating NF-κB by DAPK2, as indicated by luciferase reporter assay (Figure 5E). Collectively, our findings suggested that the activating effect of DAPK2 on NF-κB is mediated through induction of autophagy-dependent I-κBα degradation.
Discussion
Recently, the loss of DAPK expression in multiple human cancers has sparked intense interest in this kinase family. DAPK2 was originally identified as a tumor suppressor, which is lost in several cancers, such as AML, epithelial ovarian cancer, and breast cancer, due to hypermethylation of the DAPK promoter region or micro RNA (miRNA)-mediated mRNA degradation (13-15). Increased thyroid hormone signaling leads to induction of DAPK2 which suppresses the development of HCC (12). In contrast with other malignancies, we reveal that DAPK2 is upregulated in TC. Knockdown of DAPK2 in TTA1 cells led to decreased cell proliferation, clonogenic formation in vitro, and tumor formation ability in nude mice, and enhanced sensitivity to TRAIL-induced apoptosis; meanwhile overexpression of DAPK2 exhibited the opposite effect, indicating an oncogenic role of DAPK2 in TC. Unlike DAPK2, another DAPK family member, DAPK1, is downregulated and functions as a tumor suppressor in TC (16-18), suggesting that DAPK2 and DAPK1 may have divergent functions in TC development and progression. Both DAPK1 and DAPK2 can be activated by binding of calcium-activated Calmodulin to their calmodulin auto-regulatory domains (19,20). Alternatively, both can be activated by Ser289 phosphorylation, which leads to a conformational change mimicking the effect of calmodulin binding (21). Yet, Ser289 phosphorylation of DAPK1 and DAPK2 are regulated by mutually-exclusive and different signaling pathways. While DAPK1 Ser289 phosphorylation is mediated specifically by ribosomal s6 kinase (RSK) following MAPK activation (22), DAPK2 is phosphorylated on Ser289 by the metabolic sensor AMP-activated protein kinase (AMPK) (21), which is also up-regulated in TC. The different activation mode of these two similar kinases suggest the disparate roles of DPAK1 and DAPK2 in response to different stimuli (23), providing a perspective to selectively target DAPK2 in TC.
Autophagy contributes to tumor progression by maintaining the survival of tumor cells in starvation and hypoxic conditions and also confers therapeutic resistance to cancer cells (24,25). DAPK2 has been shown to be involved in several steps of autophagy. DAPK2 promotes autophagy induction by phosphorylating the mammalian target of rapamycin (mTOR) binding partner raptor, thus inhibiting mTOR complex 1 (mTORC1) activity (26). DAPK2 also activate the Beclin-1/Vacuolar protein sorting 34 (Vps34) complex through phosphorylating Beclin-1 on Thr119 and promoting its dissociation from B-Cell Lymphoma-extra-large (Bcl-XL) (21). Recently, DAPK2 was shown to interact with the Beclin-1 complex member Autophagy-related protein 14 (Atg14) (27). In addition, DAPK2 phosphorylates the autophagy receptor protein p62 to promote selective autophagy (12).
Accumulating evidence has begun to clarify the complex interplay between autophagy and NF-κB pathway, and developing new strategies that target both pathways may help improve the effectiveness of anticancer therapy. NF-κB signaling can either activate autophagy by regulating the transcription of Beclin 1 (28) or inhibit autophagy through activating mTOR (29). NF-κB signaling also controls the level of autophagy activators, such as reactive oxygen species (ROS) and oxidative stress (30,31). In turn, autophagy can either inactivate NF-κB by degradation of IkB kinase (IKK) or maintain the persistent activation of NF-κB through clearance of I-κBα (32).
NF-κB contributes to TC development and progression by maintaining the transformed phenotype and conferring resistance to apoptosis. Inhibition of constitutively activated NF-κB signaling in cancer cells may improve the efficacy of chemo- and radiotherapy. Although constitutive activation of NF-κB has been observed in various types of malignancies, genetic alterations in NF-κB members and regulatory signaling components are rare in solid tumors, suggesting cancer cells have evolved other mechanisms to activate NF-κB (33,34). I-κBα is a cytoplasmic inhibitor of NF-κB and is essential to retaining NF-κB in the cytoplasm. Extracellular stimuli could activate IKK to phosphorylate and degrade I-κBα, releasing NF-κB to induce the transcription of target genes (35,36). Constitutive degradation of I-κBα or loss-of-function mutations of I-κBα may lead to persistent NF-κB activation, while inhibition of I-κBα degradation promotes apoptotic cell death and inhibits tumorigenesis (37-39).
The autophagy and ubiquitin proteasome system (UPS) are the two major intracellular protein degradation systems, and have been thought to be largely distinct. While proteasomal degradation of I-κBα is essential for the initial induction of NF-κB activation, persistent activation of NF-κB is maintained through autophagy-dependent degradation of I-κBα (32). Our results showed that DAPK2 positively regulates degradation of I-κBα through autophagy, thus enhancing NF-κB activation. In this way, DAPK2 contributes to TC development and progression. P62 (also known as SQSTM1) is a multiple-domain protein, which binds to ubiquitinated proteins and LC3 simultaneously, mediating selective degradation of the ubiquitinated proteins by autophagy, thus coupling UPS and the autophagy-dependent degradation system (40,41). DAPK2 has also been shown to regulate autophagic clearance of ubiquitinated aggregates through phosphorylating p62 (12), suggesting p62 is probably involved in DAPK2-mediated I-κBα degradation and NF-κB activation during TC development and progression. Clearly, this study unravels an important role of DAPK2 in TC development and progression. Yet, our understanding is still in its infancy, the exact mechanism remains to be fully elucidated.
Conclusions
The present study indicates that DAPK2-driven selective autophagy promotes I-κBα degradation, activating NF-κB, and promoting TC development in turn. Thus, this study reveals a mechanistic link between autophagy and NF-κB activation, providing a novel target for TC therapy.
Acknowledgments
Funding: The authors would like to acknowledge the Clinical Research Program of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYLJ202016), the Science and Technology Commission of Shanghai Municipality (No. 19140904102 and 16401933200), the Shanghai Pulmonary Hospital Young Eagles Program (No. fkcy1910), and the National Natural Scientific Foundation of China (No. 81503579) for grant support.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-2062
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-2062
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-2062). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine institutional ethics committee (No. SH9H-2020-T346-1) and informed consent was taken from all the patients. Animal experiments were performed under a project license (No. SH9H-2020-T346-1) granted by institutional ethics committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, in compliance with Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sprague BL, Warren Andersen S, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control 2008;19:585-93. [Crossref] [PubMed]
- Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature 2004;432:1032-6. [Crossref] [PubMed]
- Netea-Maier RT, Kluck V, Plantinga TS, et al. Autophagy in thyroid cancer: present knowledge and future perspectives. Front Endocrinol (Lausanne) 2015;6:22. [Crossref] [PubMed]
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13:184-99. [Crossref] [PubMed]
- Pacifico F, Mauro C, Barone C, et al. Oncogenic and anti-apoptotic activity of NF-kappa B in human thyroid carcinomas. J Biol Chem 2004;279:54610-9. [Crossref] [PubMed]
- Mitsiades CS, McMillin D, Kotoula V, et al. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab 2006;91:4013-21. [Crossref] [PubMed]
- Yang Y, Liu YP, Bao W, et al. RNA sequencing analysis reveals apoptosis induction by hydrogen treatment in endometrial cancer via TNF and NF-κB pathways. Transl Cancer Res 2020;9:3468-82. [Crossref]
- Festa M, Petrella A, Alfano S, et al. R-roscovitine sensitizes anaplastic thyroid carcinoma cells to TRAIL-induced apoptosis via regulation of IKK/NF-kappaB pathway. Int J Cancer 2009;124:2728-36. [Crossref] [PubMed]
- Pacifico F, Leonardi A. Role of NF-kappaB in thyroid cancer. Mol Cell Endocrinol 2010;321:29-35. [Crossref] [PubMed]
- Li X, Abdel-Mageed AB, Mondal D, et al. The nuclear factor kappa-B signaling pathway as a therapeutic target against thyroid cancers. Thyroid 2013;23:209-18. [Crossref] [PubMed]
- Bretz JD, Mezosi E, Giordano TJ, et al. Inflammatory cytokine regulation of TRAIL-mediated apoptosis in thyroid epithelial cells. Cell Death Differ 2002;9:274-86. [Crossref] [PubMed]
- Chi HC, Chen SL, Tsai CY, et al. Thyroid hormone suppresses hepatocarcinogenesis via DAPK2 and SQSTM1-dependent selective autophagy. Autophagy 2016;12:2271-85. [Crossref] [PubMed]
- Rizzi M, Tschan MP, Britschgi C, et al. The death-associated protein kinase 2 is up-regulated during normal myeloid differentiation and enhances neutrophil maturation in myeloid leukemic cells. J Leukoc Biol 2007;81:1599-608. [Crossref] [PubMed]
- Zhang J, Liu L, Sun Y, et al. MicroRNA-520g promotes epithelial ovarian cancer progression and chemoresistance via DAPK2 repression. Oncotarget 2016;7:26516-34. [Crossref] [PubMed]
- Su CM, Wang MY, Hong CC, et al. miR-520h is crucial for DAPK2 regulation and breast cancer progression. Oncogene 2016;35:1134. [Crossref] [PubMed]
- Hoque MO, Rosenbaum E, Westra WH, et al. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab 2005;90:4011-8. [Crossref] [PubMed]
- Hu S, Liu D, Tufano RP, et al. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer 2006;119:2322-9. [Crossref] [PubMed]
- Khatami F, Larijani B, Heshmat R, et al. Meta-analysis of promoter methylation in eight tumor-suppressor genes and its association with the risk of thyroid cancer. PLoS One 2017;12:e0184892 [Crossref] [PubMed]
- Shohat G, Spivak-Kroizman T, Cohen O, et al. The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem 2001;276:47460-7. [Crossref] [PubMed]
- Shani G, Henis-Korenblit S, Jona G, et al. Autophosphorylation restrains the apoptotic activity of DRP-1 kinase by controlling dimerization and calmodulin binding. EMBO J 2001;20:1099-113. [Crossref] [PubMed]
- Shiloh R, Gilad Y, Ber Y, et al. Non-canonical activation of DAPK2 by AMPK constitutes a new pathway linking metabolic stress to autophagy. Nat Commun 2018;9:1759. [Crossref] [PubMed]
- Anjum R, Roux PP, Ballif BA, Gygi SP, Blenis J. The tumor suppressor DAP kinase is a target of RSK-mediated survival signaling. Curr Biol 2005;15:1762-7. [Crossref] [PubMed]
- Shiloh R, Bialik S, Kimchi A. Ser289 phosphorylation activates both DAPK1 and DAPK2 but in response to different intracellular signaling pathways. Cell Cycle 2019;18:1169-76. [Crossref] [PubMed]
- Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol 2010;21:683-90. [Crossref] [PubMed]
- Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev 2011;21:113-9. [Crossref] [PubMed]
- Ber Y, Shiloh R, Gilad Y, et al. DAPK2 is a novel regulator of mTORC1 activity and autophagy. Cell Death Differ 2015;22:465-75. [Crossref] [PubMed]
- Gilad Y, Shiloh R, Ber Y, et al. Discovering protein-protein interactions within the programmed cell death network using a protein-fragment complementation screen. Cell Rep 2014;8:909-21. [Crossref] [PubMed]
- Copetti T, Demarchi F, Schneider C. p65/RelA binds and activates the beclin 1 promoter. Autophagy 2009;5:858-9. [Crossref] [PubMed]
- Djavaheri-Mergny M, Amelotti M, Mathieu J, et al. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. The Journal of biological chemistry 2006;281:30373-82. [Crossref] [PubMed]
- Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 2010;6:838-54. [Crossref] [PubMed]
- Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 2009;11:777-90. [Crossref] [PubMed]
- Colleran A, Ryan A, O’Gorman A, et al. Autophagosomal IkappaB alpha degradation plays a role in the long term control of tumor necrosis factor-alpha-induced nuclear factor-kappaB (NF-kappaB) activity. J Biol Chem 2011;286:22886-93. [Crossref] [PubMed]
- Chaturvedi MM, Sung B, Yadav VR, et al. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene 2011;30:1615-30. [Crossref] [PubMed]
- Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol 2010;2:a000109 [Crossref] [PubMed]
- Xu L, Schüler R, Xu C, et al. Arachidonic acid inhibits the production of angiotensin-converting enzyme in human primary adipocytes via a NF-kappaB-dependent pathway. Ann Transl Med 2020;8:1652. [Crossref] [PubMed]
- Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev 2012;246:125-40. [Crossref] [PubMed]
- Cabannes E, Khan G, Aillet F, et al. Mutations in the IkBa gene in Hodgkin’s disease suggest a tumour suppressor role for IkappaBalpha. Oncogene 1999;18:3063-70. [Crossref] [PubMed]
- Jungnickel B, Staratschek-Jox A, Brauninger A, et al. Clonal deleterious mutations in the IkappaBalpha gene in the malignant cells in Hodgkin’s lymphoma. J Exp Med 2000;191:395-402. [Crossref] [PubMed]
- Palombella VJ, Rando OJ, Goldberg AL, et al. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 1994;78:773-85. [Crossref] [PubMed]
- Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007;282:24131-45. [Crossref] [PubMed]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009;137:1001-4. [Crossref] [PubMed]


