
Saving critically ill COVID-19 patients with mechanical circulatory support
Introduction
Since November 2019, coronavirus disease 2019 (COVID-19) (1,2), which arose from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (3), has had a profound effect on human society. The ongoing global pandemic that resulted from this infectious disease has rigorously challenged our ability to control viral infections. Several independent studies undertaken at different hospitals have identified the fatality risk of COVID-19 to be 2.84–15% among hospitalized cases (4-7). However, in a large number of undetected, relatively mild infections, the fatality risk could be below 1% or even below 0.1% (8). Although current research shows that some therapeutics assist in treating COVID-19 (9-11), we still require large-scale clinical trials of these treatments to confirm their effectiveness and safety (12). At this point, no specific antiviral treatment for COVID-19 is currently available. The absence of an effective pharmacological treatment able to reduce the viral load and minimize the disease’s progression to acute respiratory distress syndrome (ARDS) is one of the main factors contributing to the disease’s high mortality rate.
Given the clinical experience in treating fulminant myocarditis (FM) (13) and ARDS (14) with life support systems such as mechanical circulatory support (MCS), we believe this approach should also be considered for critically ill COVID-19 patients. Previous studies have advocated using MCS to stabilize patients experiencing circulatory and respiratory failure and to improve outcomes in high-risk populations (15,16). However, it is still unknown what factors predict weaning success and survival after MCS. Therefore, the present study investigates the efficiency of using MCS support to optimize the outcomes for COVID-19 patients. We present the following article following the STROBE reporting checklist (available at https://dx.doi.org/10.21037/atm-20-5169).
Methods
Study design and participants
In this retrospective cohort study, we selected consecutive patients with laboratory-confirmed COVID-19 who were treated at the Tongji Hospital of Huazhong University of Science and Technology from January 20, 2020, to April 10, 2020. These patients were diagnosed according to the World Health Organization’s interim guidance.
The study was conducted following the Declaration of Helsinki (as revised in 2013). This study was approved by the National Health Commission of China and the institutional review board at Tongji Hospital (Wuhan, China) (No.: TJ-IRB20200229). Written informed consent was waived by the ethics committee of the designated hospital for patients with emerging infectious diseases.
Data collection
Twenty five patients undergoing MCS were diagnosed with COVID-19 and classified as critically ill according to the Guidance for Corona Virus Disease 2019 (8th edition) released by the National Health Commission of China. Their clinical electronic medical records were reviewed and clinical data were also collected, including medical history, underlying chronic diseases, exposure history, demographics, laboratory findings, and computed tomographic scans of the chest. Furthermore, we recorded what treatments had been used since their admission to the hospital, including antiviral therapy, antibiotics, corticosteroid therapy, oxygen support, and MCS, as well as the clinical outcomes of these treatments. The clinical data were reviewed and extracted by experienced physicians who entered the data into a computerized database.
Each patient’s demographic (age, sex, body mass index), initial clinical characteristics, and date of symptom onset were collected at ICU admission. In addition, within 24 hours of each patient’s admission, their disease-severity scores [sequential organ failure assessment (SOFA) score, simplified acute physiology score (SAPS) II, and acute physiology and chronic health evaluation (APACHE) II] were assessed. All data were independently reviewed and entered into a computer database by two analysts (PC and SH).
MCS
Percutaneous extracorporeal membrane oxygenation system (ECMO)
A standard ICU single room was used during the implementation of ECMO. All staff involved were supplied with personal protective equipment following biosafety level 3. Percutaneous arterial and venous cannulation was done using the modified Seldinger technique in all patients. Cannula size was individually determined, with the average drainage cannula size being 18 Fr. for female patients and 20 Fr. for male patients. Intravenous heparin was used for anticoagulation with the adjusted activated clotting time-controlled to a range of 180–200 seconds by testing the activated partial thromboplastin time (APTT), which was maintained at 60–80 seconds.
Intra-aortic balloon pumping (IABP)
In a standard sterilized ICU single room, each patient was implanted with 8-French Datascope® catheters, and the Datascope® 2000 IABP system (Datascope Co., Fairfield, NJ, USA) was used following the modified Seldinger technique. 30 mL balloon counterpulsation was used for patients 152–163 cm tall, while 40 mL balloon counterpulsation was used for those with a height of 152–163 cm. With the help of ultrasonic localization, the balloon was oriented towards the descending aorta (1–2 cm) using the ECG trigger or pressure trigger mode. Counterpulsation was performed at a ratio of 1:1. The heparin was used to prevent thrombosis on the balloon surface, by which the ACT was maintained between 200 and 300 seconds.
Continuous renal replacement therapy (CRRT)
Taking creatinine (Cr) levels as the baseline of renal function, all patients were assessed and considered suitable for bedside catheter insertion depending on their Cr at the time of hospital admission. The staging of AKI was also defined according to the KDIGO criteria. The physician criteria were used to determine CRRT. Catheter insertion was performed at the bedside and according to International Society of Peritoneal Dialysis (ISPD) guidelines (17).
Outcomes
The initial primary outcome was patient mortality at hospital. For example, acute myocardial injury was determined if serum troponin I (TnI) levels were above the 99th percentile upper reference limit. Liver dysfunction was diagnosed when the serum concentrations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) were above the upper limit of each serum’s respective reference range (ALT ≤41 U/L, AST ≤40 U/L). Renal insufficiency was defined as eGFR ≤90 mL/min/1.73 m2. All these laboratory values were measured using equipment at the hospital. For each patient, we also recorded how long they spent in both ICU and the hospital, the total number of days they spent on mechanical ventilation (MV), and the number of in-ICU complications, including any severe hemorrhages, cannula infections, and if a patient required CRRT.
Statistical analysis
Descriptive statistics were obtained for all study variables. Continuous variables were compared using either the t-test or the Mann-Whitney U test where appropriate. To analyze all categorical variables, the Fisher exact test or χ2 test was used. These continuous data were then expressed as mean (SD) or median [interquartile range (IQR)] values. Where necessary, survival data were analyzed using the Kaplan-Meier plot or Cox proportional hazards regression model. All probability values were two‐sided, and P<0.05 was considered to be a significant statistical difference. Analysis of these statistics was performed using the statistical analysis for social science (SPSS) tool (version 13.0) (SPSS Inc., Chicago, IL, USA) for Windows (Microsoft Corp., Redmond, WA, USA).
Results
Comparison of clinical characteristics between survivors and non-survivors with MCS
A total of 25 patients [median age, 60 (IQR, 49–69) years; 80% male] were included in this study (Table 1). The most common comorbidities were hypertension (13, 52%), diabetes (9, 36%), and CHD (7, 28%), while the most common symptom was fever during/after hospitalization (80%), followed by a dry cough (56%), and dyspnoea (42.8%). There were no great differences in the symptoms of survivors and non-survivors. However, we noted that before MCS treatment, the duration of symptom onset in non-survivors [median, 26 (IQR, 21–31) days] was significantly longer than that of the survivors [median, 20 (IQR, 19–24) days]. The same type of treatment was used for all 25 patients and included antiviral, antibacterial, glucocorticoid, and respiratory support, including MV and prone position ventilation (Table 1). There was no significant difference in how survivors and non-survivors responded to the antiviral and antibacterial therapy during hospitalization. However, the response rate to RRT was much higher in survivors (11, 85%) compared with non-survivors (4, 33%). The overall duration of a patient’s hospital stay was also much longer for survivors [median, 40 (IQR, 37–58) days] than for non-survivors [median, 13 (IQR, 12–32) days].
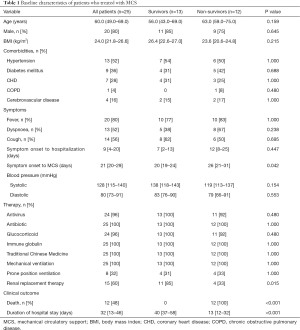
Full table
Our laboratory findings are shown in Table 2. These findings show that compared with the non-survivors, those who survived after receiving MCS showed a higher median lymphocyte count [median (IQR), 0.87 (0.59–1.69)×109/L vs. 0.59 (0.46–0.77)×109/L cells/µL], and lower levels of C-reactive protein (CRP) [median (IQR), 43.5 (35.9–94.1) vs. 128.6 (76.4–192.5) mg/dL], at the time of their admission. During hospitalization, the non-survivors also showed a higher peak cardiac troponin I (cTnI) level [474.6 (IQR, 153.3–9,210.5) vs. 113.0 (IQR, 79.9–282.4) pg/mL, P=0.03], and Interleukin 6 (IL-6) level [451.7 (IQR, 139.0–3,525.0) vs. 100.0 (IQR, 74.15–451.75) pg/mL, respectively, P=0.03], compared to the survivors.
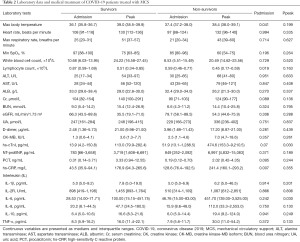
Full table
Table 3 details the mechanical support-related data of the COVID-19 patients. Compared with the group of non-survivors, those that survived at the time of MCS were characterized by lower lactate [2.12 (IQR, 1.63–2.59) vs. 3.93 (IQR, 2.28–7.52)], higher pH [7.47 (IQR, 7.37–7.55) vs. 7.22 (IQR, 7.01–7.42), P=0.014, respectively], as well as a longer duration undergoing ECMO [18 (IQR, 14–33) vs. 7 (IQR, 1–17), P=0.03], and MV [35 (IQR, 15–39) vs. 8 (IQR, 3–21), P=0.004, respectively].
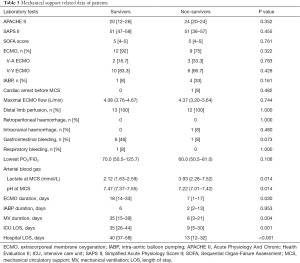
Full table
Clinical outcomes of MCS in COVID-19 patients
In our study, the survival rate of COVID-19 patients on/after MCS was 52%. Table 4 lists the variables that were tested to determine univariate associations amongst those who survived. This method was conducted using Cox proportional hazards regression modeling. Receipt of RRT was associated with a higher improvement in survival [hazard ratio (HR) =0.049, 95% confidence interval (CI) =0.008 to 0.305]. ECMO duration days and pH at the time of MCS were also associated with an increased survival rate. The HR, also at the time of MCS, for ECMO duration per day was 0.847 (95% CI =0.753–0.953) and for pH per 1 mmol/L was 0.536 (95% CI =0.297–0.971), while high-sensitivity C-reactive protein (hs-CRP) and lactate were associated with decreased survival. The HR for hs-CRP per 1 mmol/L was 1.012 (95% CI =1.003–1.022) and for lactate at MCS per 1 mmol/L 1.382 (95% CI =1.134–1.679).
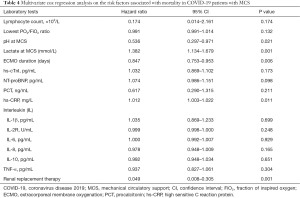
Full table
Determinants of surviving COVID-19 on/after MCS
Our study further assessed the determinants of surviving COVID-19 through MCS. We did this by comparing the inflammatory cytokine levels between the survivors and non-survivors at the time of their admission and on the 1st, 2nd, and 3rd day of treatment following MCS. This is shown in Figure 1. From admission to day 3, there was a significant increase in levels of IL-6 (β=0.009, P=0.006), IL-8 (β=0.031, P=0.020) and TNF-α (β=0.107, P=0.014) in the non-survivor group, whereas there was a non-significant decrease in IL-6, IL-8, and TNF-α levels in the survivor group. We found that most of the inflammatory cytokine levels were higher at each different time point in the group of non-survivors. There was also a significant increase in the level of lactate (β=0.173, P=0.014) and hs-CRP (β=0.125, P=0.008) in the non-survivors, while there was a non-significant change in levels of pH during ECMO, as well as in NT-proBNP proteins, in the survivors (Figure 2).
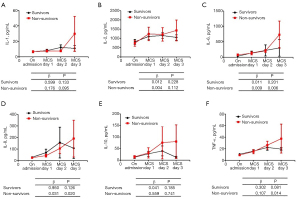
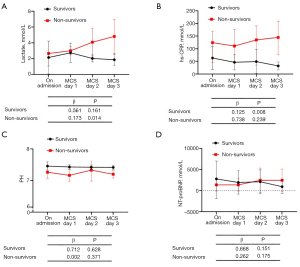
Discussion
Our study systematically evaluated the impact of MCS on the clinical characteristics and prognosis of patients diagnosed with COVID-19 in China. The major findings of this study include: (I) MCS should be implemented before the onset of multiple organ dysfunction syndromes (MODS) induced by respiratory failure, hypoxemia, and inflammatory storms, instead of used as a salvage therapy; (II) the study found there was a 52% survival rate of COVID-19 patients who were weaned from MCS; and (III) excessive myocardial injury, the release of inflammatory cytokines, and the prolonged time spent on MCS are important clinical determinants of successful weaning from MCS.
An increasing amount of reports have shown that advanced age (>60), male sex, and comorbidities (particularly hypertension) are believed to be associated with high mortality from the SARS-Cov-2 infection (6,18). Based on current data, the overall mortality rate of COVID-19, despite its high rate of infectivity, is much lower than severe acute respiratory syndrome (SARS) (10%) and Middle East respiratory syndrome (MERS) (30%). However, due to many COVID-19 patients having atypical symptoms during the early stages of the infection, and because of its high infectivity, COVID-19 has ultimately proven more deadly (19). Here, we have reported a relatively high mortality rate (up to 48%) for critically ill COVID-19 patients treated with MCS, which is higher than in recent reports (6). Furthermore, among the non-survivors, the timing of our intervention (from symptom onset to MCS) was noted to be significantly later than that experienced by the survivors. As we know, there is still no specific drug treatment for COVID-19, but early intervention for critically ill patients can result in a better prognosis. Therefore, through this study, we suggest that MCS should be implemented at the onset of MODS induced by respiratory failure, hypoxemia, and inflammatory storms.
Increasing evidence has shown that COVID-19 patients with comorbidities have poorer clinical outcomes (20). This was certainly the case for our study, as most of the patients treated had one or more complications, and almost all were admitted to the hospital with multiple organ damage. Abnormalities in all these markers suggested severe inflammatory storms and multiple organ failure. If these risk factors are not addressed quickly and effectively, they can lead to a higher chance of patient mortality. Indeed, we can see that the degree of organ damage was significantly higher in the patients with MCS who died than in the patients who survived. In addition, the Extracorporeal Life Support Organization (ELSO) registry demonstrated that early MCS deployment before respiratory or circulatory failure might be associated with better outcomes (21). Even so, severe ARDS is associated with a mortality rate that can exceed 40% (22). In our study, the survival rate of COVID-19 patients treated with MCS was approximately 52%.
Another interesting finding in this study was the significant reduction in mortality among patients treated with CRRT. Increasing clinical data shows that approximately 3–7% of patients with COVID-19 develop acute kidney injury. Among these patients, the proportion who received CRRT was 7–9%, and this proportion in the ICU was as high as 23% (18,23). From this we can deduce that CRRT contributes to clinical outcomes in critically ill COVID-19 patients. The potential advantages of CRRT in the treatment of COVID-19 patients includes: (I) maintaining a patient’s internal stability, e.g., through correcting and maintaining the water, electrolyte, and acid-base balance; (II) improving metabolic disorders in the body; (III) exerting effective treatment for volume overload; (IV) improving inflammation, endothelial function, and immune status.
Despite these findings, the factors which influence prognosis and lead to surviving COVID-19 following MCS are still poorly understood. Previous studies have shown that the timing of peak troponin levels was an important predictor of myocardial recovery (13,24). Our study shows that continued MCS therapy and RRT may have good outcomes for critically ill patients with COVID-19. This may be due to 80% of our patients undergoing kidney replacement therapy, which helped improve their internal environment and clear out inflammatory factors. Despite this fact, only 3.2% of COVID-19 patients were treated with kidney replacement therapy in the New York City Area, with just 21% of those patients noted to have died (25). Mortality for those requiring mechanical ventilation was also identified to be 88.1%. From this, we can discern that the successful treatment of COVID-19 requires collaboration between medical staff from across a diverse range of fields.
For this reason, the Chinese government has recruited more than 40,000 medical staff from all over the country to come to Wuhan to learn about COVID-19 treatments. These staffs include personnel from respiratory departments, cardiovascular departments, infection departments, critical medicine departments, and other fields. This is an important factor that will influence Wuhan’s success in the treatment of COVID-19.
Study limitations
This study carried with it several limitations which should be clarified. Firstly, this was an observational study, and we cannot draw clear markers of cause-and-effect between laboratory findings, treatments, and mortality of COVID-19 patients. Secondly, COVID-19 is a systemic disease that can cause damage to multiple organs throughout the body. Therefore etiology-based therapies, as well as multidisciplinary therapies, still need to be developed. Lastly, our conclusions should be treated carefully as the outcomes of using MCS to treat COVID-19 need to be confirmed through larger studies and across multiple areas.
Conclusions
This case study analyzes the characteristics and outcomes of sequentially hospitalized critically ill COVID-19 patients in the Wuhan area.
From our observations, we have found that inflammatory cytokines, pH, lactate, hs-CRP, ECMO duration, and RRT are important clinical determinants for successful weaning from MCS.
Acknowledgments
The authors would like to thank C.J who helped with data collection. We also want to acknowledge all the participants in this study.
Funding: This work was supported by the grant and key project from the National Natural Science Foundation of China (Nos. 81770351 and 81630010) and the National Precision Medicine Program (No. SQ2017YFSF090157).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/atm-20-5169
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-20-5169
Peer Review File: Available at https://dx.doi.org/10.21037/atm-20-5169
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-20-5169). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the National Health Commission of China and the institutional review board at Tongji Hospital (Wuhan, China) (No.: TJ-IRB20200229). Written informed consent was waived by the ethics commission of the designated hospital for patients with emerging infectious diseases.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fung TS, Liu DX. Human Coronavirus: Host-Pathogen Interaction. Annu Rev Microbiol 2019;73:529-57. [Crossref] [PubMed]
- Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol 2020;18:123. [Crossref] [PubMed]
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565-74. [Crossref] [PubMed]
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [Crossref] [PubMed]
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-10. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol 2020;92:441-7. [Crossref] [PubMed]
- Wu P, Hao X, Lau EHY, et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill 2020; [Crossref] [PubMed]
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72-3. [Crossref] [PubMed]
- Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 2020;156:104761 [Crossref] [PubMed]
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269-71. [Crossref] [PubMed]
- Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020;382:1787-99. [Crossref] [PubMed]
- Matsumoto M, Asaumi Y, Nakamura Y, et al. Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Fail 2018;5:675-84. [Crossref] [PubMed]
- Hardin CC, Hibbert K. ECMO for Severe ARDS. N Engl J Med 2018;378:2032-4. [Crossref] [PubMed]
- Asaumi Y, Yasuda S, Morii I, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J 2005;26:2185-92. [Crossref] [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Cullis B, Abdelraheem M, Abrahams G, et al. Peritoneal dialysis for acute kidney injury. Perit Dial Int 2014;34:494-517. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547 [Crossref] [PubMed]
- Diddle JW, Almodovar MC, Rajagopal SK, et al. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med 2015;43:1016-25. [Crossref] [PubMed]
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:1904-5. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Hsu KH, Chi NH, Yu HY, et al. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: analysis of a single center's experience. Eur J Cardiothorac Surg 2011;40:682-8. [Crossref] [PubMed]
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323:2052-9. [Crossref] [PubMed]


