
Siderophores induce mitophagy-dependent apoptosis in platelets
Introduction
Klebsiella pneumoniae carries many kinds of virulent factors, and its infections will induce severe inflammatory response and organ dysfunction (1,2). Platelets, a type of anucleate blood cells, play critical roles in hemostasis and thrombosis and take part in inflammatory and immunological response (3,4). K. pneumoniae infections alter the functions of platelets and reduce platelet count of infected patients (5). Lipopolysaccharides were the major virulent factor of K. pneumoniae, and it has been reported that lipopolysaccharides can stimulate platelet secretion and potentiate platelet aggregation (6). Also, lipopolysaccharides were reported to induce apoptosis of platelets (7). Some other virulent factors of K. pneumoniae apart from lipopolysaccharides may have some impacts on platelets, including siderophores.
Siderophores are a small, high-affinity iron-chelating molecule and can be secreted by many gram-negative bacteria (8). K. pneumoniae strains can secret four kinds of siderophores, enterobactin, yersiniabactin, salmochelin, and aerobactin (9). These four siderophores have higher affinity for iron than transferrin, and the central roles of these siderophores are competitors for serum iron to promote bacterial growth. As a critical regulator of mitochondrial biogenesis, iron takes part in the synthesis of iron-sulfur clusters and the work of an electron transport chain. A decrease of iron will result in mitochondrial dysfunction, further increase the production of reactive oxygen species (ROS) and result in cell apoptosis (10). Some studies have shown that iron chelation can induce cell apoptosis in some tumor cells (10-13). Besides, a recent study pointed out that loss of iron could trigger mitophagy in skin fibroblasts, which was independent on the PINK1/Parkin signal pathway (14). Mitophagy is a specific form of autophagy, assisting to selectively remove dysfunctional or superfluous mitochondria in cells, and disorder of mitophagy can remove healthy mitochondria and further result in many diseases. Platelets are capable of synthesizing new proteins, although without nuclei and autophagic machinery is constitutively present in platelets (15). We hypothesize that secreted siderophores can induce apoptosis and mitophagy of platelets after K. pneumoniae infections. However, to date, there is no related study, and the impacts of siderophores on platelets are still unknown.
In this study, we performed in vitro experiments to determine whether siderophores secreted by K. pneumoniae can induce apoptosis and mitophagy of platelets. Furthermore, the role of mitophagy in platelets was explored in our study as well. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4861).
Methods
Reagents and antibodies
Bafilomycin A1 (Baf A1), 3-methyladenine (3-MA) and desferrioxamine (DFO) were bought from Sigma Aldrich. Enterobactin, yersiniabactin, salmochelin, and aerobactin were bought from EMC micro collections. The following antibodies were used for western blot: rabbit polyclonal anti-LC3 (Cell Signaling Technology), rabbit polyclonal anti-p62 (ProteinTech), rabbit polyclonal anti-TOMM20 (Cell Signaling Technology), rabbit polyclonal anti-TIMM23 (Cell Signaling Technology), rabbit polyclonal anti- PTEN-induced putative kinase 1 (PINK1) (Cell Signaling Technology) and rabbit polyclonal anti-BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) (Cell Signaling Technology). Rabbit polyclonal anti-LC3 was further used for immunofluorescence staining. Anti-TUBA antibody and DAPI were bought from BD for immunofluorescence staining. Mitotracker was bought from Cell Signaling Technology. JC1 mitochondrial membrane potential (MMP) assay kit and H2DCF-DA were bought from BD. Recombinant lipocalin 2 (Lcn2) protein was bought from Novoprotein, China.
Platelet preparation
Venous blood was drawn from healthy volunteers by standard venipuncture and collected into tubes holding 4% sodium citrate (w/v). Whole blood was centrifuged at 200 ×g for 10 min, and 75% of supernatant was collected to obtain platelet-rich plasma. Then platelets were sedimented at 1,000 ×g for 10 min and resuspended in calcium-free modified Tyrode’s solution. After sedimentation, the platelets were washed twice using the same solution and resuspended at 2×108–3×108/mL in the solution.
The study was approved by Affiliated Hospital of Jiangnan University (No. 201800416) and written informed consent was obtained from all volunteers. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Siderophore stimulation experiments
Washed platelets were resuspended in Tyrode’s solution and incubated with the indicated combinations of 10 µM ammonium ferric citrate, 5, 10, 25 or 50 µM enterobactin, 10 µM yersiniabactin, 10 µM salmochelin, 10 µM aerobactin, 10 µM DFO, or 10 µM Lcn2 for 2 hours. Platelets were incubated with 0.5 µM Baf A1 or 3 mM 3-MA for 1 hour before incubation with the reagents mentioned above in some groups of these experiments. Siderophore-iron-Lcn2 complexes were prepared, as described previously (16). Before incubation with platelets, ferric citrate and siderophore were incubated at room temperature for 30 minutes. The complexes were then sequentially incubated with Lcn2 for an added 30 minutes.
Western blot
Standard Western blot assay protocols were used in this study. In brief, twenty micrograms of protein from lysed platelets were resolved on SDS-PAGE gels and then transferred to PVDF membranes. The membranes were blocked in 5% bovine serum albumin and then stained with corresponding primary antibodies (1:500). We analyzed the band intensity using Image-pro plus analysis software after incubated with the corresponding secondary antibodies. We finally converted the intensity value to fold change, yet with the intervention group and the control group.
Flow cytometry
ROS level and MMP changes were measured by flow cytometry. ROS levels were measured using H2DCF-DA, and changes in the MMP were measured using a JC1 MMP assay kit (BD, USA), respectively. Both factors were analyzed using flow cytometry (BD FACSCalibur, USA). Both experiments were performed in triplicate.
Immunofluorescence assay
For immunofluorescence assay, 25 µL of incubated platelets were attached to pre-treated adhesion slides. Standard immunofluorescence assay protocols were applied in this study. Briefly, the platelets were fixed with pre-cooled methanol for 30 minutes, after which the slides were washed twice in phosphate-buffered saline (PBS). Mitochondria were then stained using MitoTracker for 15 min, and slides were washed twice for 5 min in PBS. The slides were permeabilized for 20 min in 0.5% Triton X-100 and then washed three times in PBS. After that, the slides were blocked for 30 min in 10% bovine serum albumin at room temperature and incubated in 3% primary antibody overnight at 4 °C. Then the slides were washed twice in PBS again and additionally incubated for 1 h at room temperature in 3%secondary antibodies. Antibodies for LC3 were used. The stained platelets were observed using a Carl Zeiss laser scanning confocal microscopy. Five sets of images were obtained for each group.
Statistics
SPSS 22 was used for all data analyses. All the statistical data were expressed as the means with standard deviations and analyzed using ANOVA analysis. P<0.05 were statistically significant.
Results
Enterobactin induced mitophagy of platelets
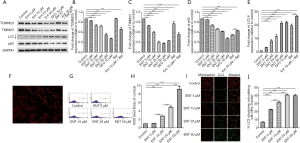
Enterobactin is the most common siderophore in K. pneumoniae. The in vitro experiments were initially performed to determine whether enterobactin can induce mitophagy in platelets by checking the expression of TOMM20 and TIMM23, which exists in the outer and inner membrane of mitochondria, and LC3 and p62, which are the key markers of autophagosome and autophagy substrate, respectively. Platelets were freshly prepared, and the expression of TOMM20, TIMM23, LC3, and p62 were analyzed with specific antibodies by western blotting. Mitophagy in platelets was induced by the treatment of enterobactin. Mitochondrial membrane proteins, TOMM20, and TIMM23 decreased following the stimulation of enterobactin (Figure 1A,B,C). The expression of LC3-II increased along with the increased concentration of enterobactin compared with the control group, and the treatment of enterobactin plus Baf A1 resulted in the accumulation of LC3-II compared with the treatment of enterobactin only (Figure 1D). Expression of p62 in platelets decreased after the stimulation of enterobactin, and it significantly increased after treated with enterobactin plus Baf A1 than that treated with Baf A1 only (Figure 1E). Also, DFO, a prevalent iron chelator, showed a stronger ability to induce platelet mitophagy than enterobactin in our study. Washed platelets were stained with anti-TUBA antibody and DAPI, as shown in Figure 1F, showing the purity of platelets. ROS was an essential factor to induce mitochondrial dysfunction. Thus, here we measured the ROS level in distinct groups by flow cytometry. The results showed that enterobactin increased ROS level in platelets, as shown in Figure 1G,H. We then sought to demonstrate mitophagy flux by measuring the co-localization of the mitochondrial marker mitotracker with the autophagy marker LC3-II. The treatment of enterobactin significantly increased the co-localization of mitotracker with LC3-II, as shown in Figure 1I,J. Notably, platelet mitochondria were damaged in 50 µM enterobactin group so that mitotracker staining decreased a lot, which was associated with increased LC3.
All four siderophores secreted by K. pneumoniae induced mitophagy of platelets
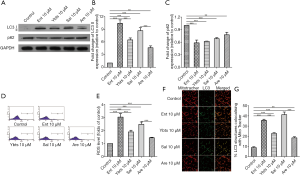
Some K. pneumoniae strains secret four kinds of siderophores, and we then treated platelets with these four siderophores to determine the impacts of them on platelets. Similarly, all treatments of these four siderophores increased the expression of LC3-II and decreased the expression of p62 in platelets (Figure 2A,B,C), despite the different change in four groups. ROS level was measured in these four groups as well, and the results showed that all four kinds of siderophores increased ROS level in platelets, as shown in Figure 2D,E. We then determined the mitophagy flux by measuring the co-localization of mitotracker with LC3-II again, which revealed the treatment of four siderophores all increased the co-localization of mitotracker with LC3-II, as shown in Figure 2F,G.
Mitophagy of platelets was activated through several pathways
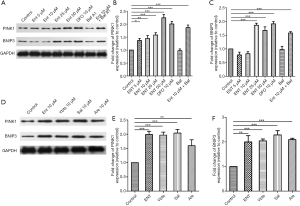
PINK1/Parkin- and BNIP3-dependent pathways were the most studied pathways of mitophagy. Here we measured the activation of the two pathways by analyzed with specific antibodies by western blotting. The result showed that both two proteins of platelets were increased after treated with siderophores (Figure 3A,B,C,D,E,F).
Lcn2 cannot reduce mitophagy induced by siderophores, and protective effects of iron differed in distinct groups
When K. pneumoniae secret siderophores into the bloodstream, neutrophils will release Lcn2 to combine with siderophores. The interactions then cut the ability of K. pneumoniae siderophores to scavenge iron by binding to its secreted siderophores. However, Lcn2 cannot combine with all kinds of siderophores. In the siderophores secreted by K. pneumoniae, Lcn2 only binds to enterobactin and passes the other three siderophores. The role of Lcn2 in the siderophore-induced mitophagy was unknown, so here we further treated platelets with Lcn2 plus enterobactin or yersiniabactin to figure out their crosstalk. The results showed that Lcn2 could not protect platelets from the stimulation of siderophores. The expression of LC3 II in platelets treated with iron or Lcn2 only increased a lot compared with the control group. When platelets were treated with enterobactin, the addition of Lcn2 cannot reduce the expression of LC3 II but increased its expression (Figure 4A,B).
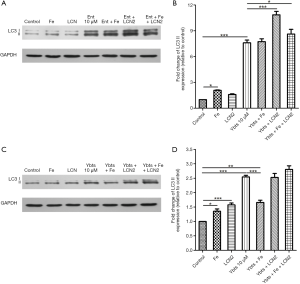
However, the addition of Lcn2 had no significant impact on LC3 II expression when platelets were treated with yersiniabactin (Figure 4C,D). We additionally treated platelets with iron to verify that activation of mitophagy in platelets after treated with siderophores was caused by iron deficiency. However, it was found the role of iron differed in different experiments. When platelets were treated with iron only, the expression of LC3 II increased compared with the control group. When platelets were treated with enterobactin plus iron, mitophagy was activated, and the expression of LC3 II was like that after the treatment of enterobactin only (Figure 4A,B). However, when platelets were treated with yersiniabactin plus iron, the expression of LC3 II decreased a lot compared with that after the treatment of yersiniabactin only (Figure 4C,D).
Siderophores induced apoptosis of platelets
To find the impacts of siderophores on apoptosis of platelets, we then measured the expression of caspase-3 in platelets after treated with different concentrations and kinds of siderophores by analyzing with specific antibodies by western blotting. The results showed that the expression of caspase-3 increased in platelets when the treated concentration of enterobactin increased (Figure 5A,B). Flow cytometry measured MMP to confirm the apoptosis of platelets in distinct groups, and the results indicated a higher concentration of enterobactin induced more apoptosis of platelets (Figure 5C). The other three kinds of siderophores also induced increased caspase-3 of platelets, in which aerobactin induced most, followed by salmochelin, yersiniabactin, and enterobactin (Figure 5D,E). MMP measurements also showed that all four siderophores induced apoptosis of platelets (Figure 5F).
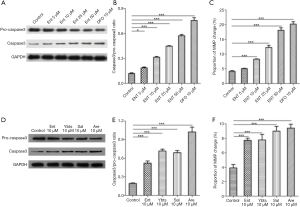
Apoptosis of platelets induced by siderophores is partly dependent on mitophagy
The last question remained as to the role of siderophores-induced mitophagy in platelets. 3-MA is the inhibitor of the PI3K pathway, the primary pathway of autophagy. We here used 3-MA to inhibit the mitophagy of platelets. Then we continued to treated platelets with different siderophores and observed the change of platelet apoptosis. Autophagy was inhibited in platelets treated with 3-MA, as shown in Figure 6A,B. The ability to increase platelet expression of caspase3 was significantly reduced after autophagy was inhibited in siderophores, enterobactin, or yersiniabactin, as shown in Figures 6A,C. The proportion of MMP change was decreased in platelets treated with 3-MA (Figure 6D).

Discussion
Our study, for the first time, investigated the impacts of K. pneumoniae siderophores on platelets. K. pneumoniae is a common pathogen rapidly acquiring multiple drug resistance. A detailed understanding of all K. pneumoniae virulent factors is beneficial to develop novel therapies for K. pneumoniae infections. Siderophores are important virulent factors of K. pneumoniae, and we used human platelets to perform in vitro experiments to determine the impacts of siderophores on platelets. The results showed that siderophores induced apoptosis and mitophagy of platelets, and mitophagy induced by siderophores played a harmful role in platelets, aggravating platelet apoptosis. These findings supplied a new understanding of coagulant function abnormality in severe K. pneumoniae infections.
K. pneumoniae can generate four kinds of siderophores to scavenge iron from the host. In our study, the mitophagy of platelets was induced by these siderophores. Allen et al. have reported that loss of iron would lead to the loss of mitochondria respiration and trigger mitophagy of fibroblasts (14). Our study showed that treatments of siderophores induced the generation of ROS in platelets. Accumulation of mitochondrial dysfunction and generated ROS are harmful to cells. Several studies have reported that the accumulation of mitochondrial ROS increased the expression of HIF-1alpha and its target genes BINP3 and NIX (BINP3L), which later triggered mitophagy (17-19). Also, iron chelators were found to induce the degradation of ferritin (20). Mitophagy was activated after treated with siderophores to resolve mitochondria and release a large amount of iron to support the balance of iron metabolism. The release of iron can also support essential iron-dependent functions. Mitophagy, as we know, is regulated through two kinds of distinct signaling pathways in mammalian cells, PINK1/Parkin-dependent pathway, and receptor-mediated pathways (21). The most studied receptor-mediated pathway is BNIP3-dependent pathways. In our study, both two pathways in platelets were activated after treated with siderophores. Different mitochondrial stresses activate different mitophagy pathways. Mitochondrial damage and depolarization are the leading cause of Parkin-dependent pathway activation (22,23). BNIP3 exists in the outer mitochondrial membrane of mitochondria and has a role in hypoxia-induced mitophagy (24). Siderophores produce multiple impacts on platelets and mitochondria. On the one hand, siderophores chelate iron of platelets and impair mitochondrial respiratory chain, resulting in damage and depolarization of mitochondria. However, several studies have reported siderophores secreted by K. pneumoniae can stabilize hypoxia-inducible factor-1α (25,26), which was the primary activator of BNIP3-pathway.
Lcn2, also called neutrophil gelatinase-associated lipocalin, siderocalin or 24p3, was secreted by neutrophils and host mucosal cells to counter the iron-scavenging effects of siderophores. Lcn2 binds to enterobactin, either in its ferric or aferric form, in the binding pocket to neutralize enterobactin. We initially hypothesized Lcn2 could protect platelets from the stimulation of enterobactin by reducing iron loss. However, the result is contrary to our hypothesis. When platelets were treated with enterobactin plus Lcn2, the expression of LC3 II in platelets even increased compared with that in platelets treated with enterobactin only. It was confusing to observe such results, but we found some explanations afterward. As we know, Lcn2 limits bacterial iron acquisition by binding to ferric enterobactin and preventing it from releasing iron to K. pneumoniae strains. Lcn2 has a stronger affinity to ferric enterobactin, which means it cannot prevent enterobactin from chelating iron in our study. Also, the enterobactin-iron-Lcn2 complex further has some added impacts on the host. Song et al. found that siderophore-iron-Lcn2 complex increased mitochondrial ROS generation and attenuated mitochondrial oxidative phosphorylation in adult rat primary cardiomyocytes (27). Lcn2 also has been reported to activate autophagy in kidney cells with ischemia/reperfusion damage (28-30), which was like the results that the expression of LC3 II in platelets increased after treatment of Lcn2 only. From here, we see that Lcn2 is a double-edged sword for the host. On the one hand, it binds siderophores to reduce cellular iron loss and limit bacterial iron acquisition.
However, it increases mitochondrial ROS generation and aggravated cell damage. The other three siderophores cannot be bound by Lcn2. Thus, the expression of LC3 II in platelets did not change after the treatment of yersiniabactin plus Lcn2 compared with the treatment of yersiniabactin only.
The affinity of K. pneumoniae siderophores for iron varies with distinct types of siderophores. It was reported that enterobactin had the highest affinity for iron, and aerobactin had the lowest (31). The different iron affinity of siderophores results in different abilities of siderophores to induce mitophagy, as shown in Figure 2. The addition of iron also has different impacts on platelets treated with different siderophores due to different affinities of siderophores. In our study, the expression of LC3 II in platelets treated with enterobactin plus iron was like that in platelets treated with enterobactin only.
Meanwhile, the expression of LC3 II in platelets treated with yersiniabactin plus iron decreased a lot compared with that in platelets treated with yersiniabactin only. It can be explained that enterobactin chelate more iron than yersiniabactin so that the addition of iron has no significant effects on platelets treated with enterobactin. Each enterobactin molecule can chelate three iron ions, and each yersiniabactin molecule can chelate only one iron ion. However, the ratio of siderophore and iron concentration was 1:1 in our study, so most of the enterobactin still did not get iron, while only a few of yersiniabactin did not get iron.
The mitophagy change of platelets after treated with siderophore plus iron and Lcn2 is hard to explain. As we see, the treatment of iron or Lcn2 only also induced the mitophagy of platelets. It was reported that iron overload promoted mitochondrial fragmentation in mesenchymal stromal cells through activation of the AMPK pathway (32), showing the addition of iron may be an activator of mitophagy. Therefore, considering so many confounding factors, including siderophores, Lcn2, iron, siderophore-iron complex, and siderophore-iron-Lcn2 complex, one more targeted study is still needed.
Moreover, our study found that all these four siderophores can induce apoptosis of platelet via the classic caspase-3 pathway. Iron depletion has been reported to be associated with apoptosis of some tumor cells by exerting their antiproliferative activity (10-13). Several studies have proposed that iron chelators can be potential novel therapeutic agents for the treatment of cancer (33,34). Shirazi et al. also showed apoptosis induced Rhizopus oryzae when it grew in iron-depleted media (35). However, the mechanism, through which iron chelators induce cell apoptosis, is still not clear to date. In gastric cancer cells, iron chelators induced ROS production and then activated both JNK and endoplasmic reticulum stress apoptotic pathways (10). Also, Iron deficiency is a risk factor for manganese accumulation through the upregulation of divalent metal transporter 1 (36). Accumulated manganese further induced cell apoptosis through endoplasmic reticulum stress and later unfolded protein response pathway (36). The endoplasmic reticulum stress pathway may be the significant apoptotic pathway activated by iron chelators, but other mechanisms should be explored further. Besides, the abilities of different siderophores to induce platelet apoptosis were significantly different in our study, which cannot be explained by different affinities of siderophores. Aerobactin induced the most platelet apoptosis in all. However, its affinity for iron was lowest. We hypothesized aerobactin itself might have some impacts on platelets. There is no related study to date, and this is what we want to explore afterward.
Usually, mitophagy acts as a safeguard mechanism against cell apoptosis. Many studies have reported that mitophagy would counteract apoptosis in distinct types of cells (37-39). However, recently Chen et al. proposed that autophagy protein LC3B can activate extrinsic apoptosis during cigarette smoke-induced emphysema (40). Another study also revealed the expression and activity of caspase-3 decreased in dynamin-1-like protein (DRP1, a fundamental part of mitochondrial fission and further facilitate mitophagy)-knockdown cells. Our study also showed that the apoptosis of platelets was dependent on mitophagy induced by siderophores. If mitophagy was inhibited, platelet apoptosis was alleviated. Here we proposed mitophagy of platelets induced by siderophores contributes to platelet apoptosis, which can be a potential therapeutic target to treat thrombocytopenia in K. pneumoniae infections.
In summary, our study firstly found that all four kinds of K. pneumoniae siderophores can induce apoptosis and mitophagy of platelets by chelating iron. The mitophagy in platelets was activated through several pathways, including PINK1/Parkin and BNIP3 pathway. Lcn2 cannot reduce platelet mitophagy induced by siderophores, whereas, Lcn2 binding to ferric enterobactin promoted mitophagy of platelets. Furthermore, apoptosis of platelets was partly dependent on mitophagy, and inhibition of mitophagy would suppress the apoptosis of platelets induced by siderophores.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4861
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4861
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4861). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Affiliated Hospital of Jiangnan University (No. 201800416) and written informed consent was obtained from all volunteers.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiao Y, Qin Y, Liu J, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health 2015;109:68-74. [Crossref] [PubMed]
- Gu B, Bi R, Cao X, et al. Clonal dissemination of KPC-2-producing Klebsiella pneumoniae ST11 and ST48 clone among multiple departments in a tertiary teaching hospital in Jiangsu Province, China. Ann Transl Med 2019;7:716. [Crossref] [PubMed]
- Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost 2009;7:1759-66. [Crossref] [PubMed]
- Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264-74. [Crossref] [PubMed]
- Li L, Huang H. Risk factors of mortality in bloodstream infections caused by Klebsiella pneumonia: A single-center retrospective study in China. Medicine (Baltimore) 2017;96:e7924. [Crossref] [PubMed]
- Zhang G, Han J, Welch EJ, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol 2009;182:7997-8004. [Crossref] [PubMed]
- Nocella C, Carnevale R, Bartimoccia S, et al. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb Haemost 2017;117:1558-70. [Crossref] [PubMed]
- Holden VI, Bachman MA. Diverging roles of bacterial siderophores during infection. Metallomics 2015;7:986-95. [Crossref] [PubMed]
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev 2016;80:629-61. [Crossref] [PubMed]
- Kim JL, Lee DH, Na YJ, et al. Iron chelator-induced apoptosis via the ER stress pathway in gastric cancer cells. Tumour Biol 2016;37:9709-19. [Crossref] [PubMed]
- Moon JH, Jeong JK, Park SY. Deferoxamine inhibits TRAIL-mediated apoptosis via regulation of autophagy in human colon cancer cells. Oncol Rep 2015;33:1171-6. [Crossref] [PubMed]
- Choi JG, Kim JL, Park J, et al. Effects of oral iron chelator deferasirox on human malignant lymphoma cells. Korean J Hematol 2012;47:194-201. [Crossref] [PubMed]
- Sun X, Ge R, Cai Z, et al. Iron depletion decreases proliferation and induces apoptosis in a human colonic adenocarcinoma cell line, Caco2. J Inorg Biochem 2009;103:1074-81. [Crossref] [PubMed]
- Allen GF, Toth R, James J, et al. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep 2013;14:1127-35. [Crossref] [PubMed]
- Feng W, Chang C, Luo D, et al. Dissection of autophagy in human platelets. Autophagy 2014;10:642-51. [Crossref] [PubMed]
- Bachman MA, Oyler JE, Burns SH, et al. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun 2011;79:3309-16. [Crossref] [PubMed]
- Basit F, van Oppen LM, Schockel L, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis 2017;8:e2716. [Crossref] [PubMed]
- Hu L, Wang H, Huang L, et al. The Protective Roles of ROS-Mediated Mitophagy on (125) I Seeds Radiation Induced Cell Death in HCT116 Cells. Oxid Med Cell Longev 2016;2016:9460462. [Crossref] [PubMed]
- Wei X, Qi Y, Zhang X, et al. ROS act as an upstream signal to mediate cadmium-induced mitophagy in mouse brain. Neurotoxicology 2015;46:19-24. [Crossref] [PubMed]
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood 2009;114:4546-51. [Crossref] [PubMed]
- Williams JA, Ding WX. Mechanisms, pathophysiological roles and methods for analyzing mitophagy-recent insights. Biol Chem 2018;399:147-78. [Crossref] [PubMed]
- Matsuda N, Sato S, Shiba K, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010;189:211-21. [Crossref] [PubMed]
- Kane LA, Lazarou M, Fogel AI, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol 2014;205:143-53. [Crossref] [PubMed]
- Band M, Joel A, Hernandez A, et al. Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J 2009;23:2327-35. [Crossref] [PubMed]
- Holden VI, Breen P, Houle S, et al. Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1alpha Stabilization during Pneumonia. mBio 2016;7:e01397-16. [Crossref] [PubMed]
- Holden VI, Lenio S, Kuick R, et al. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1alpha and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect Immun 2014;82:3826-36. [Crossref] [PubMed]
- Song E, Ramos SV, Huang X, et al. Holo-lipocalin-2-derived siderophores increase mitochondrial ROS and impair oxidative phosphorylation in rat cardiomyocytes. Proc Natl Acad Sci U S A 2018;115:1576-81. [Crossref] [PubMed]
- Yan C, Yuanjie T, Zhengqun X, et al. Neutrophil Gelatinase-Associated Lipocalin Attenuates Ischemia/Reperfusion Injury in an In Vitro Model via Autophagy Activation. Med Sci Monit 2018;24:479-85. [Crossref] [PubMed]
- Zhang W, Yang S, Cui L, et al. Neutrophil gelatinase-associated lipocalin worsens ischemia/reperfusion damage of kidney cells by autophagy. Ren Fail 2016;38:1136-40. [Crossref] [PubMed]
- Jin D, Zhang Y, Chen X. Lipocalin 2 deficiency inhibits cell proliferation, autophagy, and mitochondrial biogenesis in mouse embryonic cells. Mol Cell Biochem 2011;351:165-72. [Crossref] [PubMed]
- Brock JH, Williams PH, Liceaga J, et al. Wooldridge KG. Relative availability of transferrin-bound iron and cell-derived iron to aerobactin-producing and enterochelin-producing strains of Escherichia coli and to other microorganisms. Infect Immun 1991;59:3185-90. [Crossref] [PubMed]
- Zheng Q, Zhao Y, Guo J, et al. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells from myelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis 2018;9:515. [Crossref] [PubMed]
- Kamihara Y, Takada K, Sato T, et al. The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/beta-catenin signaling in human multiple myeloma. Oncotarget 2016;7:64330-41. [Crossref] [PubMed]
- Brard L, Granai CO, Swamy N. Iron chelators deferoxamine and diethylenetriamine pentaacetic acid induce apoptosis in ovarian carcinoma. Gynecol Oncol 2006;100:116-27. [Crossref] [PubMed]
- Shirazi F, Kontoyiannis DP, Ibrahim AS. Iron starvation induces apoptosis in Rhizopus oryzae in vitro. Virulence 2015;6:121-6. [Crossref] [PubMed]
- Seo YA, Li Y, Wessling-Resnick M. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 2013;38:67-73. [Crossref] [PubMed]
- Yan H, Xiao F, Zou J, et al. NR4A1-induced increase in the sensitivity of a human gastric cancer line to TNFalpha-mediated apoptosis is associated with the inhibition of JNK/Parkin-dependent mitophagy. Int J Oncol 2018;52:367-78. [PubMed]
- Shen YQ, Guerra-Librero A, Fernandez-Gil BI, et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J Pineal Res 2018;64. [Crossref] [PubMed]
- Swiader A, Nahapetyan H, Faccini J, et al. Mitophagy acts as a safeguard mechanism against human vascular smooth muscle cell apoptosis induced by atherogenic lipids. Oncotarget 2016;7:28821-35. [Crossref] [PubMed]
- Chen ZH, Lam HC, Jin Y, et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci U S A 2010;107:18880-5. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


