Targeted temperature management at 33 degrees Celsius in patients with high-grade aneurysmal subarachnoid hemorrhage: a protocol for a multicenter randomized controlled study
Introduction
Subarachnoid hemorrhage (SAH) is an uncommon type of stroke in which bleeding in the subarachnoid space leads to brain injury, high rates of morbidity, and death (1). SAH is most often caused by the rupture of an intracranial aneurysm (85% of cases), but can less frequently be idiopathic or the result of various other causes (1-3). The global reported incidence of SAH is 9.1 cases per 100,000 persons/year, peaking in adults aged 50–60 years old (1,4). Modifiable risk factors for SAH include hypertension, smoking, alcohol abuse, and sympathomimetic drug use such as cocaine (1,5).
The management of patients with SAH includes determining the grade of SAH (e.g., Hunt and Hess grade) and transferring the patient to a comprehensive stroke center, when possible. Regular neurological and vital sign assessments are conducted until the patient is stable. Blood pressure (BP) must be monitored closely as normal BP is essential to avoid the risk of rebleeding. The culprit aneurysm must be repaired as soon as possible, with the aim of complete obliteration. Other considerations include maintaining euvolemia, starting nimodipine, elevating the head to 30° for at least 24–48 h, and managing acute complications such as cerebral vasospasms, ischemia, hydrocephalus, seizure, hyponatremia, fever, hyperglycemia, deep vein thrombosis, anemia, edema, neurocardiogenic injury, and renal dysfunction (5-10). However, the prognosis of SAH remains poor, with a 30-day mortality rate of 33% (11), while 80% of survivors are unable to resume their previous lifestyle in 3–6 months (12) and 45% unable to regain independent function (1).
Therapeutic hypothermia (TH) may improve several outcomes in both ischemic strokes and SAH (13-15), although it may not reduce intracranial pressure (ICP) (16-19). The exact mechanisms of TH protection could include reducing the neuronal metabolic rate, reducing oxidative stress, alleviating inflammation, inhibiting excitotoxicity, and inhibiting apoptosis (20). Also, a reduction in blood flow velocity could play a role in improving patient outcomes when high ICP is present (21). Two studies have suggested that TH can be used in patients with poor-grade SAH and that it could reduce the occurrence of cerebral vasospasm and delayed cerebral ischemia (22-25), although this is not supported by others (26-29). Among the latter, a retrospective study by Karnatovskaia et al. (26) showed that the use of TH (n=19) with decompressive hemicraniectomy (DHC) in patients with aSAH, global cerebral edema, and refractory high ICP prolonged the duration of mechanical ventilation and failed to improve the Rankin score compared with DHC alone (n=16). Seule et al. (27) suggested that prolonged TH should be kept as a last-resort option for patients with SAH and high ICP because of safety issues. A recent meta-analysis suggested that TH did not reduce mortality and poor outcomes, but decreased delayed cerebral ischemia occurrence (30). While high-quality trials on TH for SAH are missing, the ongoing “Cooling in IntraCerebral Hemorrhage” randomized controlled trial could provide insightful results in the future (31). Another ongoing randomized controlled trial, “HYBERNATUS” (32), plans to enroll 270 patients with convulsive status epilepticus, randomized to TH vs. standard care. A review highlighted that the conflicting results among trials could be due to the different TH protocols used and that special care should be taken during rewarming (33).
Indeed, despite its potential benefits, TH carries some risks that must be tightly kept in check. During the induction phase, the fluid balance must be verified to prevent hypovolemia and hypotension; glucose and electrolyte must be maintained at the proper levels; infections, pressure injuries, and shivering have to be prevented (33,34). During the maintenance phase, pneumonia, infections, and pressure injury should be prevented (33,34). During rewarming, there is a risk of hypokalemia and imbalances in oxygen supply/consumption (35,36). In general, TH carries risks of ischemia due to coronary vasoconstriction, electrolyte abnormalities, hyperglycemia, impaired blood gases, mild acidosis, bleeding, infection (>50% risk of nosocomial pneumonia), respiratory alkalosis and hypocapnia, cold diuresis, shivering, and altered drug metabolism that can increase the risk of drug-related adverse events (37).
The purpose of this trial is to evaluate the safety and efficacy of TH (maintaining bladder temperature at 33 °C for ≥3 days) for the treatment of patients with high-grade (Hunt-Hess grade IV–V) aSAH. All patients in this study will be followed for 6 months after SAH to evaluate the efficacy and safety of TH in the treatment of high-grade aSAH.
We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4719).
Methods
Study design
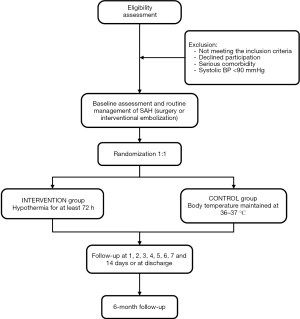
A prospective, multicenter, randomized, controlled clinical trial is proposed for October 2020 to September 2024 involving 10 clinics. Patients who meet the inclusion criteria are randomized 1:1 to a TH group and a normal temperature group (Figure 1). The trial will enroll 96 participants in TH group and normothermia one, respectively. The trial was registered with ClinicalTrials.gov (NCT03442608) on February 22, 2018. This study was approved by the ethics committee of Xuanwu Hospital ([2019]032) and will follow the tenets of the Declaration of Helsinki (as revised in 2013).
Study population
The inclusion criteria are: (I) 18–65 years of age, male or non-pregnant female; (II) SAH confirmed by plain CT scan, mFisher grade 3–4, global cerebral edema, Hunt-Hess grade IV-V, WFNS grade IV–V; (III) aneurysm confirmed by CTA or DSA and responsible for SAH; (IV) admission within 72 h after bleeding; (V) Glasgow coma scale (GCS) 3–8 points at admission or after resuscitation; (VI) Aneurysms responsible for SAH should be treated by surgical clamping or interventional embolization as soon as possible when the patient’s condition permits after admission or after resuscitation; (VII) an intracranial pressure probe was implanted in the operating room or ICU; and (VIII) the subject or their legal representative understood and signed informed consent documentation.
The exclusion criteria are: (I) serious underlying organ diseases such as severe heart disease or other intracranial diseases; (II) systolic BP <90 mmHg after resuscitation; (III) bilateral fixed and dilated pupils.
The standard procedure is to secure the aneurysm first, and then randomize the patients.
Randomization and blinding
Center-based stratification is used, and blocked randomization is conducted within each center using a computer generated random sequence. The random coding scheme is distributed in envelopes and sent to each center, assigning an individual to be responsible for the custody of the envelopes. The custodian does not participate in the process of inclusion, grouping, and treatment of the patients, and when a patient meets the criteria, the clinician notifies the custodian and requests an allocation number. The patients will be included during working hours, night shifts and weekend ones. And there will be a 24-h hotline for the physicians if there are questions on patient inclusion.
Treatment and management
All patients undergo conventional neurosurgical treatment protocols and are treated in the Neuro-ICU, Stroke Unit or General ICU after surgery. The main principles refer to the “Consensus on Management of Severe Aneurysmal Subarachnoid Hemorrhage (2015)” (38). The main goals are to maintain a systolic BP of 120–160 mmHg, oxygen saturation ≥95%, intracranial pressure <25 mmHg using ICP probe monitoring, and cerebral perfusion pressure (CPP) of at least 50 mmHg. The latter is measured with an MAP transducer at the level of the foramen of Monro during the first 72 hours after aneurysm securing. After 72 hours, a CPP of 70–80 mmHg may be required because of vasospasm. Antihypertensive drugs include urapidil, nicardipine, and nimodipine. Extracranial spinal fluid drainage, 20% mannitol and/or 4.2% hypertensive saline are used to reduce intracranial pressure. Transcranial Doppler ultrasound (TCD) is performed daily, and cerebral vasospasm is indicated when blood flow velocity >120 cm/s in the middle cerebral artery (MCA) or the ratio of blood flow velocity in the MCA to that in the internal carotid >3. Routine blood and urine examination, chest X-ray are performed before starting the cooling procedure.
The TH group is treated with TH (bladder temperature maintained at 33 °C) plus conventional neurosurgery management. TH includes intravascular cooling, analgesics, and sedatives. An 8.5F (outer diameter is 2.87 mm) × 38 cm heat exchange catheter (Icy catheter, Alsius, USA) is inserted from the femoral vein to the inferior vena cava. The heat exchange catheter consists of an infusion lumen and a saline lumen (three cooling balloons that can circulate isotonic saline). The infusion lumen is used to infuse of fluids or medicine, and the saline lumen is in full contact with the blood for heat exchange. The heat exchange catheter is connected to an external heat exchange controller (CoolGard 3000, Alsius, USA). A temperature-measuring silicone urethral catheter (Guangzhou Weili Medical Equipment Co., Ltd.) is used to monitor the bladder temperature of the patient, and the probe connected to the heat exchange controller. The heat exchange controller can automatically adjust the temperature of the saline cavity based on the bladder temperature. When inducing TH, a maximum rate of 2 °C/h is used to reduce the bladder temperature to a targeted temperature 33 °C as soon as possible, then maintained for at least 72 hours. Appropriate doses of sedatives and analgesics are used to prevent shivering when implementing TH. The Bedside Shivering Assessment Scale (39) is used to asses shivering. The use of midazolam 0.02–0.1 mg/kg/h, propofol 4–12 mg/kg/h, dexmedetomidine 0.2–0.7 µg/kg/h, remifentanil 0.02–0.15 µg/kg/h, meperidine 0.03–0.06 mg/kg/h, respectively is recommended. The speed and dosage of analgesics and sedatives are based on the patient’s body core temperature, BP, heart rate stability, and presence of shivering, and a ventilator should be used to prevent respiratory depression caused by their use. TH is maintained for ≥72 hours. Rewarming is performed slowly after the intracranial condition improves. The rewarming rate should not exceed 0.5 °C/4 h. During rewarming, care is taken to keep the other vital indicators stable. If a patient gets severe vasospasm during rewarming, a temperature of 33 °C is maintained for several days until relief of severe vasospasm. If severe pneumonia, septic shock, bleeding due to coagulation dysfunction, or heart failure occur during TH, rewarming should be initiated in advance.
In the normal temperature group, the bladder temperature is maintained at 36–37 °C. The temperature measurement method is the same as in the TH group. When the patient is admitted to hospital, hyperthermic treatment is not performed if there is a low body temperature. When the body temperature is >37 and <38.5 °C, the temperature is lowered with ice packs. When the body temperature is >38.5 °C, acetaminophen and indomethacin, cold iv. fluids, and ice blanket are used to lower the temperature. If the fever is refractory to this medication, adjunctive cooling measures such as cooling blanket and ice packs are applied to maintain bladder temperature at 36–37 °C. Sedative and analgesic drugs should be given when necessary. The cause of fever must be investigated by physical, laboratory and imaging examination. Physical examination includes pulmonary auscultation et al. Laboratory examination includes blood routine test, PCT, blood culture, sputum culture, cerebrospinal examination and so on. Imaging examination includes chest X-ray, chest CT and so on. We usually perform chest X-ray once three days. Antibiotics would be given according to the clinical situation after blood and urine cultures.
Safety
Adverse events (AEs) such as vasospasm, pneumonia, and deep vein thrombosis are recorded during treatment. Severe adverse events (SAEs) are defined as events requiring hospitalization, prolonged hospital stays, impacting on work capacity, life-threatening or death, resulting in disability, congenital malformation, and other events. When an SAE occurs, the investigator must immediately take necessary measures to protect the safety of the patient. The “adverse reaction monitoring team” or the general medical duty team should immediately contact the relevant department to assist in the rescue and the patient sent to the ICU if necessary.
Course of events
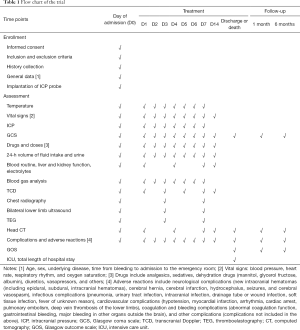
The examinations to be performed and data to be collected are outlined in Table 1. The vital signs are evaluated after admission to the emergency department. If unstable, resuscitation is performed immediately. The GCS score, BP, and SPO2 are evaluated, and after the patient’s vital signs are stabilized, a skull CT examination is performed for the first time. DSA is performed after the diagnosis is confirmed, and surgery or interventional embolization is performed after an aneurysm diagnosis is confirmed. The necessity of implanting an ICP probe is explained to family members, and the probe is implanted in the operating room or ICU. After returning to the ward, the patient is admitted to the ICU or neurosurgery ICU. Randomization is performed, and TH is performed according to grouping. During treatment, the body temperature, name and dose of various drugs, input and output fluid volumes over 24 h, supplemented electrolytes, types and doses of nutritional support, complications, GCS score, ICP, blood routine, electrolytes, blood gas analysis, TCD, chest X radiographs, and ultrasound of bilateral lower limbs and other results are recorded over the 7–15 days of hospitalization. The head CT is repeated at 1, 4, and 7 days, then 1 month, and 6 months after bleeding. Telephone or outpatient follow-up is performed at 1 and 6 months after bleeding.
Full table
Withdrawal criteria
All subjects for whom a consent form is signed, undergo screening, and who qualified for the clinical trial are considered as lost, irrespective of when they were lost, if any of the following occur: (I) family members ask that the patient be withdrawn; (II) the investigator considers from a medical perspective that the subject must terminate the study; (III) those who had an incomplete informed consent process; and (IV) those who need to suspend the trial for other reasons. The cause and date of loss are collected.
Endpoints
The primary endpoint is the Glasgow outcome scale (GOS) at 6 months after bleeding. The secondary endpoints are: (I) mortality at 6 months after bleeding; (II) ICP; (III) ICU stay; and (IV) hospital stay. The safety endpoints are complications, including neurological complications (cerebral vasospasm, cerebral infarction, cerebral hemorrhage, hydrocephalus, etc.), infectious complications (intracranial infection and pneumonia), complications of coagulation and bleeding (deep limb deep vein thrombosis), complications of the circulatory system, electrolyte disorders, intestinal dysfunction, and other complications.
Sample size
A review of previous studies of hypothermia (26,31), indicated that the proportion of patients with GOS ≥4 points in the TH group at 6 months would be 40%, and the proportion in the control group will be 20%. Considering a cut-off value for the superiority of 3%, a statistical significance level (α) of 0.05, and a power (1-β) of 80%, it was calculated that 86 patients are required in each group. With an assumed loss rate of 10%, this means 96 cases are required.
Statistical analysis
This study will use a full analysis set (patients who received the treatment and one efficacy evaluation) for efficacy analysis and a safety data set (patients who received the treatment and one efficacy evaluation) for safety analysis. The missing data will be handled using the last observation carried forward method.
An interim analysis will be performed when 96 patients have completed the follow-up. Only safety indicators will be analyzed. If the proportion of complications in the TH group is significantly higher than that of the control group (chi-square test or exact probability method), the clinical trial will be terminated. A stratified analysis will be performed according to different treatment settings.
The 6-month GOS is an ordinal categorical variable. Comparisons between groups will be performed using the Mann-Whitney U-test and the unordered categorical variables, such as sex, will be analyzed with the chi-square test or exact probability method. Analysis of continuous variables such as age will be performed using the independent sample t-test or Mann-Whitney U test, according to the results of the Kolmogorov-Smirnov test for normal distribution.
Discussion
This study will provide high-quality data about the effectiveness and safety of TH in patients with high-grade aSAH. The primary endpoint is the GOS at 6 months, and the sample size is based on a proportion of patients with GOS ≥4 points in the TH group of 40% compared with 20% in the control group. Similar studies used mortality as the primary endpoint (26,31,32), which could lead to some resolution and accuracy loss. Instead of analyzing the outcomes dead/alive, the present study will examine the patients’ condition regarding the extent of improvement. We consider that early TH after SAH could reduce the occurrence of vasospasm and lead to better recovery of functional outcomes at 6 months. Nevertheless, we acknowledge that the results about the efficacy of TH are conflicting and that there is a possibility that the results of this trial will not be definitive. Despite this, the secondary endpoints of mortality at 6 months, ICP, ICU stay, hospital stay, and complications can provide useful data for the use of TH for patients with high-grade aSAH and other subgroups.
It must be highlighted that the prognosis of high-grade aSAH is poor and there is a paucity of research on the condition. If the study hypothesis is confirmed, TH will improve patient outcomes and may become part of standard practice in the treatment of aSAH. The safety of TH is a concern, and we designed criteria for the early termination of TH and interim analysis to address this. During rewarming period, the rewarming rate should not exceed 0.5 °C/4 h as too fast a rise in core body temperature could cause systemic vasodilation and hypotension, which could trigger cerebral vasodilation and rebound edema and increased ICP (40). Conversely, a too slow rewarming speed could result in more hypothermia related complications.
The results of the present study will need be carefully weighed against the available data. Indeed, Karnatovskaia et al. (26) showed that the use of TH prolonged the duration of mechanical ventilation and did not improve the mRankin score compared with DHC alone. Seule et al. (27) suggested that prolonged TH should be kept as a last-resort option for patients with aSAH. Two ongoing trials titled, “Cooling in Intracerebral Hemorrhage” (31) and “HYBERNATUS” (32), should provide further information.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81372473), National Key R&D program of China (2016YFC1300800) and Project on research and application of effective intervention techniques for high risk population of stroke from the National Health and Family Planning Commission in China (GN-2016R0004).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4719).
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4719
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-4719
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4719). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was registered with ClinicalTrials.gov (NCT03442608) on February 22, 2018. This study was approved by the ethics committee of Xuanwu Hospital ([2019]032) and will follow the tenets of the Declaration of Helsinki (as revised in 2013). All patients enrolled will complete the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet 2017;389:655-66. [Crossref] [PubMed]
- Mensing LA, Vergouwen MDI, Laban KG, et al. Perimesencephalic Hemorrhage: A Review of Epidemiology, Risk Factors, Presumed Cause, Clinical Course, and Outcome. Stroke 2018;49:1363-70. [Crossref] [PubMed]
- Lago A, Rogelio L, Jose IT, et al. Short- and long-term outcomes in non-aneurysmal non-perimesencephalic subarachnoid hemorrhage. Neurol Res 2016;38:692-7. [Crossref] [PubMed]
- Etminan N, Chang HS, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol 2019;76:588-97. [Crossref] [PubMed]
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:1711-37. [Crossref] [PubMed]
- Casaubon LK, Boulanger J, Blacquiere D, et al. Canadian Stroke Best Practice Recommendations: hyperacute stroke care guidelines, Update 2015. Int J Stroke 2015;10:924-40. [Crossref] [PubMed]
- Thompson BG, Brown RD, Amin-Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms. Stroke 2015;46:2368-400. [Crossref] [PubMed]
- Eskey CJ, Meyers PM, Nguyen TN, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation 2018;137:e661-89. [Crossref] [PubMed]
- Diringer MN, Bleck TP, Claude Hemphill JR, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011;15:211-40. [Crossref] [PubMed]
- Steiner T, Juvela S, Unterberg A, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013;35:93-112. [Crossref] [PubMed]
- Lovelock CE, Rinkel GJE, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010;74:1494-501. [Crossref] [PubMed]
- Etminan N, Macdonald RL. Management of aneurysmal subarachnoid hemorrhage. Handb Clin Neurol 2017;140:195-228. [Crossref] [PubMed]
- Han Z, Liu X, Luo Y, et al. Therapeutic hypothermia for stroke: where to go? Exp Neurol 2015;272:67-77. [Crossref] [PubMed]
- Schwab S, Georgiadis D, Berrouschot J, et al. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke 2001;32:2033-35. [Crossref] [PubMed]
- Hong JM, Lee JS, Song H, et al. Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke 2014;45:134-40. Erratum in: Stroke 2014;45:e12. [Crossref] [PubMed]
- Agrawal D. Endovascular treatment for poorest-grade subarachnoid hemorrhage in the acute stage: has the outcome been improved? Neurosurgery 2003;52:481-2. [Crossref] [PubMed]
- Karibe H, Sato K, Shimizu H, et al. Intraoperative mild hypothermia ameliorates postoperative cerebral blood flow impairment in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2000;47:594-9; discussion 599-601. [PubMed]
- Takino M, Okada Y. Hyperthermia following cardiopulmonary resuscitation. Intensive Care Med 1991;17:419-20. [Crossref] [PubMed]
- van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007;130:3063-74. [Crossref] [PubMed]
- Sun YJ, Zhang ZY, Fan B, et al. Neuroprotection by Therapeutic Hypothermia. Front Neurosci 2019;13:586. [Crossref] [PubMed]
- Seule M, Muroi C, Sikorski C, et al. Therapeutic hypothermia reduces middle cerebral artery flow velocity in patients with severe aneurysmal subarachnoid hemorrhage. Neurocrit Care 2014;20:255-62. [Crossref] [PubMed]
- Choi W, Kwon SC, Lee WJ, et al. Feasibility and safety of mild therapeutic hypothermia in poor-grade subarachnoid hemorrhage: prospective pilot study. J Korean Med Sci 2017;32:1337. [Crossref] [PubMed]
- Kuramatsu JB, Kollmar R, Gerner ST, et al. Is hypothermia helpful in severe subarachnoid hemorrhage? an exploratory study on macro vascular spasm, delayed cerebral infarction and functional outcome after prolonged hypothermia. Cerebrovasc Dis 2015;40:228-35. [Crossref] [PubMed]
- Flynn LMC, Rhodes J, Andrews PJD. Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: preliminary data from the Eurotherm3235 Trial. Ther Hypothermia Temp Manag 2015;5:143-51. [Crossref] [PubMed]
- Kochanek PM. Therapeutic hypothermia for severe traumatic brain injury. JAMA 2003;289:3007. [Crossref] [PubMed]
- Karnatovskaia LV, Lee AS, Festic E, et al. Effect of prolonged therapeutic hypothermia on intracranial pressure, organ function, and hospital outcomes among patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 2014;21:451-61. [Crossref] [PubMed]
- Seule MA, Muroi C, Mink S, et al. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery 2009;64:86-92; discussion 92-3. [Crossref] [PubMed]
- Andrews PJD, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. New Engl J Med 2015;373:2403-12. [Crossref] [PubMed]
- Sandestig A, Romner B, Grände P. Therapeutic hypothermia in children and adults with severe traumatic brain injury. Ther Hypothermia Temp Manag 2014;4:10-20. [Crossref] [PubMed]
- Yao Z, You C, He M. Effect and feasibility of therapeutic hypothermia in patients with hemorrhagic stroke: a systematic review and meta-analysis. World Neurosurg 2018;111:404-412.e2. [Crossref] [PubMed]
- Kollmar R, Juettler E, Huttner HB, et al. Cooling in intracerebral hemorrhage (CINCH) trial: protocol of a randomized German–Austrian clinical trial. Int J Stroke 2012;7:168-72. [Crossref] [PubMed]
- Legriel S, Pico F, Tran-Dinh Y, et al. Neuroprotective effect of therapeutic hypothermia versus standard care alone after convulsive status epilepticus: protocol of the multicentre randomised controlled trial HYBERNATUS. Ann Intensive Care 2016;6:54. [Crossref] [PubMed]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008;371:1955-69. [Crossref] [PubMed]
- Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Intensive Care Med 2004;30:757-69. [Crossref] [PubMed]
- Kawahara F, Kadoi Y, Saito S, et al. Slow rewarming improves jugular venous oxygen saturation during rewarming. Acta Anaesth Scand 2003;47:419-24. [Crossref] [PubMed]
- Lavinio A, Timofeev I, Nortje J, et al. Cerebrovascular reactivity during hypothermia and rewarming. Brit J Anaesth 2007;99:237-44. [Crossref] [PubMed]
- Soleimanpour H, Rahmani F, Golzari SE, et al. Main complications of mild induced hypothermia after cardiac arrest: a review article. J Cardiovasc Thorac Res 2014;6:1-8. [PubMed]
- Xu Y, Wang N, Hu J, et al. Consensus on management of severe aneurysmal subarachnoid hemorrhage. Chin J Cerebrovasc Dis 2015;12:215-25.
- Jain A, Gray M, Slisz S, et al. Shivering treatments for targeted temperature management. J Neurosci Nurs 2018;50:63-7. [Crossref] [PubMed]
- Badjatia N. Therapeutic hypothermia protocols. Handb Clin Neurol 2017;141:619-32. [Crossref] [PubMed]