
Astilbin influences the progression of osteoarthritis in rats by down-regulation of PGE-2 expression via the NF-κB pathway
Introduction
Osteoarthritis (OA) is a common chronic disease that affects middle-aged and elderly patients worldwide. It is mainly characterized by the progressive destruction of articular cartilage, synovium, and peri-articular soft tissue (1). At present, the treatment of OA includes conservative and drug therapies (2). Pro-inflammatory cytokines produced by the infiltration of inflammatory cells and chondrocytes can be detected in the synovial fluid of patients with OA. Chondrocytes are the cells in articular cartilage, which can balance the synthesis and degradation of the extracellular matrix (3). Chondrocytes obtained from OA patients will release interleukin 1 beta (IL-1β), which induces the production of metalloproteinase (MMPs), leading to cartilage degeneration (4). The inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) are essential mediators of matrix degradation, which promote the release of nitric oxide (NO) and prostaglandin E2 (PGE2), and these inflammatory mediators can further cause pain and enhance the inflammatory response (5).
Astilbin (AST), a natural flavonoid compound, is first isolated from the rhizome of Astilbe chinensis, widely used for its various pharmacological properties. It has been reported that AST has various biological activities, including anti-liver fibrosis (6), anti-oxidation (7), anti-diabetic nephropathy (8), anti-inflammation (9), anti-arthritis etc. Some studies also have shown AST can inhibit bone marrow differentiation factor 88 (MyD88), p65, and inhibitor KB kinase β (IKK β) through the NF-κB signal pathway, to treat chronic inflammatory diseases including rheumatoid arthritis (10). Also, AST has been proved to have no genotoxicity, which further shows the tremendous value of this compound in clinical application. Recent studies have shown that AST can prevent renal damage by inhibiting the expression of transforming growth factor-β (TGF-β1) and connective tissue growth factor (CTGF), and protect the kidney by inhibiting the formation of monosodium urate (MSU) and PGE2 and IL-1. These studies supply convincing evidence for AST as a safe and promising drug for the treatment of clinical diseases.
However, the efficacy and mechanism of the AST-mediated inflammatory pathway in the treatment of OA are not clear. Therefore, in this study, we used papain-induced rats to evaluate the therapeutic effect of AST on OA and further explore its possible molecular mechanism of inflammation.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4485).
Methods
AST and main reagents
Pure AST is provided by Shanghai Yuanye Biotechnology Co., Ltd. In the experiment, pure AST was dissolved with phosphate buffer (PBS) and diluted with saline. Pentobarbital sodium, papain, and cysteine were purchased from Guangzhou Chemical Reagent Factory. Hematoxylin, water-soluble eosin Y, toluidine blue O, paraformaldehyde, and Ethylene Diamine Tetraacetic Acid (EDTA) were from TAKARA, Japan. Anti- PEG2, β-actin, and other monoclonal antibodies were purchased from Abcam, UK. Finally, the immunohistochemical kit was purchased from Boster, Wuhan, China) The fluorescence quantitative PCR detection kit was purchased from Baoshi, Dalian, China, and the Prime Script RT kit were purchased from Takara, Dalian, China.
Animals
SD rats, male, 12 weeks old, 200 mL 250 g, were bought from the Experimental Animal Center of Guiyang College of Traditional Chinese Medicine. The mice are in line with the specific pathogen-free (SPF) conditions of the Animal Care and Use Committee of Guiyang College of Traditional Chinese Medicine, raised in the Experimental Animal Center of Guiyang College of Traditional Chinese Medicine and fed with routine water and food. All the experiments conducted in this study are approved by the experimental animal ethics committee of Guangdong Medical Experimental Animal Center.
The establishment and intervention of the animal model
OA model was constructed in 12-week-old rats. Firstly, the rats were randomly divided into four groups (n=24): drug group, PBS group, OA group, and control group. The rats in the drug group, PBS group, and OA group were injected with 0.25 mL/kg of a mixed solution of 4% (w/v) papain and 0.3 mol/L cysteine at the knee joint on the 1st, 3rd and 5th days, while the control group did not receive surgical intervention. After the model was successfully established in the four groups, 3 mg/kg of AST was given to the drug group by gavage, the equal volume of PBS buffer was given to PBS group by gavage, and the other two groups were treated with an equal volume of saline at the same way, once a day for 4 weeks. Four weeks after the operation, the mice were euthanized with pentobarbital sodium, and the knee joint tissue of all the mice was collected for further tests.
HE staining
We took the cartilage tissue of the knee joint of the rat out and fixed it in 4% paraformaldehyde for 24 hours and then dehydrate it with graded ethanol. Embed the waxed tissue in the embedding machine and slice the trimmed wax block on a paraffin slicer and cut it a thickness of 4 µm. After routine dewaxing, the slices were stained with hematoxylin for 8 min, washed with tap water for 1 min, and then stained with 3 min in eosin solution. Finally, the slides were sealed with gradient alcohol dehydration, transparent xylene, and neutral gum.
Toluidine blue staining
After routine dewaxing, the slices were stained with 0.5% toluidine blue solution for 30 min, washed with tap water, then soaked in 0.5% glacial acetic acid for 5 s, and then washed with distilled water. Finally, the slices were sealed with gradient alcohol dehydration, transparent xylene, and neutral gum, and the average optical density of toluidine blue was analyzed.
Detect the PGE2 with the immunohistochemical method
According to the instructions of immunohistochemical detection reagents, the slices were successively placed in xylene I for 15 min, xylene II for 15 mis, anhydrous ethanol I for 5 min, absolute alcohol 85%, 75% alcohol for 5 min, and washed with distilled water, then placed in 37 °C incubators for 30 min, rinsed with PBS for 5 min, 3 times. Then soaked in 3% hydrogen peroxide (H2O2) for 25 min, 3 times, to cut endogenous peroxidase activity. Rinsed with PBS for 5 min, 3 times. The blocking solution, 5% bovine serum albumin (BSA), was dripped and kept at room temperature for 30 min in a wet box. Wipe off the sealing fluid with filter paper rather than wash. Incubate the slice with primary antibodies of appropriate concentration in a wet box at 4 °C overnight, then wash off the primary antibody with PBS for 5 min, 3 times. Wipe off the PBS outside the sample with filter paper. The biotinylated secondary antibody solution is added to the slice and incubated in a wet box at room temperature for 50 min, with PBS for 5 min, 3 times to wash off the secondary antibody. The fresh Diaminobenzidine (DAB) solution was added to control the color development time under the microscope. Brownish-yellow is positive. Rinse the slices with tap water to stop the color development and rinse thoroughly with tap water. Then re-stain with hematoxylin for 3 min, dehydrate, transparent, and sealed with neutral gum. Image J was used to analyze the percentage of positive area in the results of immunohistochemistry.
The detection of cartilage protein expression in each group
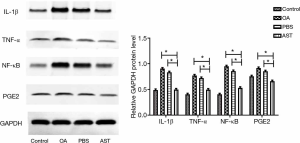
Western blot was used to detect the expression of IL-1β, TNF-α, NF-κB, and PGE2 in the synovium tissue of the keen rat joint of each group. The articular cartilage was placed in a mortar, rapidly ground after adding liquid nitrogen, after grinding, 1 g:4 mL of RIPA protein cleavage solution was added to the mortar for cracking for one time per 30 min. Two hours later, the lysate was placed in a 1.5 mL centrifuge tube, centrifuged with 14,000 r/min at 4 °C for 30 min. The 200 µL supernatant was absorbed out and placed in a 0.6 mL centrifuge tube, 50 µL protein loading buffer was added and boiled for 5 min, and samples were injected according to the quantitative results of BCA protein. Add 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to react for 2 hours, and 5% skimmed milk powder at room temperature to seal for 2 hours. Then the add β-catenin antibody, IL-1β (1:1,000), TNF-α (1:1,000), NF-κB (1:1,000) and PGE2 (1:1,000) respectively as primary antibody and incubated overnight at 4 °C. Wash with TBST washing buffer for 10 min, 3 times. Add goat anti-rabbit IgG/HRP antibody 4 mL as the secondary antibody, incubated for 1 hour at room temperature, wash with the washing buffer of TBST for 10 min, 3 times, and then developed through air exposure. The gray-scale value was analyzed by gel graphic analysis Image Lab, and the relative expression of the protein was calculated.
Real-time fluorescence quantitative PCR
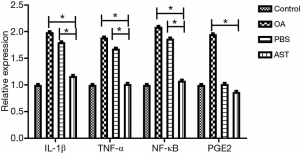
The total mRNA of rat cartilage tissue was extracted by TRIzol reagent. According to the instructions, mRNA was reverse transcribed with the PrimeScript RT kit, and then real-time PCR was conducted by using a Thermal Cycler Dice real-time system with SYBR Green I dye. In this study, we designed sequence-specific primers to produce products between 104 and 421 bp in length, which were listed in Table 1. The mean Ct value of the genes studied was standardized as GAPDH, and the results were quantified by RQ (2−∆∆CT). All experiments were repeated at least three times.
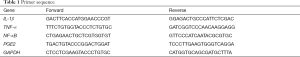
Full table
Statistical processing
The data were obtained from at least three independent experiments and expressed with mean ± standard deviation. Single-factor analysis of variance (ANOVA) and t-test were adopted for comparison between groups. The data were analyzed by SPSS 20.0 (SPSS Company, Chicago, Illinois, USA). When P<0.05, it was considered that the difference was statistically significant.
Results
HE staining analysis
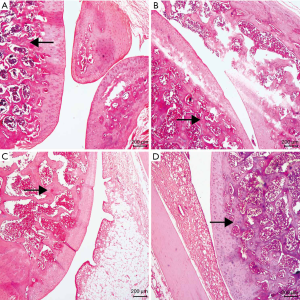
The articular cartilage of the drug group has a relatively smooth surface, neatly arranged cells, clear four-layer structure, intact tidal line, normal matrix staining, and the moderate thickness of cartilage. Meanwhile, in the PBS group, compared with the drug group, there were fewer chondrocytes, the disordered arrangement of cells, light staining of the cartilage matrix, discontinuous tidal line, and unclear four-layer structure. Compared with the drug group, the OA group had irregular cartilage surfaces, cracks, the disordered arrangement of cells, light staining of the cartilage matrix, and loss of tidal line. However, in the control group, the cartilage surface was smooth, the arrangement of cells was regular, and the cartilage matrix was moderately discolored (see Figure 1). Among them, the drug group has the most significant improvement, and the least joint damage, which is close to the results of the control group, and the chondrocytes are neatly arranged, relative to PBS, with less cell damage.
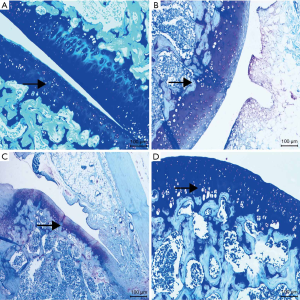
Toluidine blue staining
The toluidine blue staining section showed that the cartilage in the drug group was blue-purple with relatively uniform staining. Meanwhile, the color depth was higher than that in the OA group and the PBS group. The PBS group had broken the structure and the rough surface layer, where the staining became light. Additionally, the staining depth and area in the OA group were lower than those in the PBS group and the drug group, and an extensive area showed a loss of staining in some parts, showing light blue. Furthermore, the content of proteoglycan was significantly lower in other groups. In the control group, the upper part of the cartilage was blue-purple, with dark staining, large area, deep and uniform staining (see Figure 2).
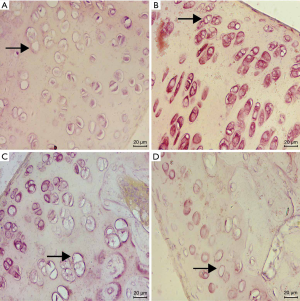
Immunohistochemical results
The drug group had light staining and no apparent brown expression. In the PBS group and the OA group, a substantial number of brown staining can be observed, while almost no brown staining was observed in the control group. Compared with the control group, the expression of PGE2 in the knee joint tissue of the model group and the drug group increased. Meanwhile, compared with the model group, the expression of PGE2 in the knee joint tissue of the drug group decreased in varying degrees (see Figure 3, Table 2).
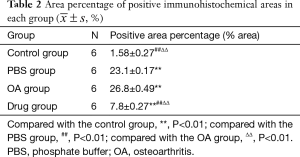
Full table
Western blot
Western Blot detection suggested the protein contents of IL-1β, TNF-α, PGE2, and NF-κB in cartilage tissue of rats in the drug group were significantly lower than those in the PBS group and the OA group (P<0.01). Compared with the PBS and OA group, the relative expression of each protein in the control group decreased, yet the decrease in IL-1β and NF-κB was smaller than that in the drug group (see Figure 4).
PCR
The results of RT-PCR indicated that compared with the PBS group and the OA group, the mRNA levels of IL-1β, TNF-α, and NF-κB in the cartilage of rats in the drug group were significantly lower, with statistically significant difference (P<0.05). The levels of PEG2 in the cartilage tissue in the drug group were not significantly lower than those in the PBS group, whereas the mRNA level of PEG2 in the drug group was significantly declined compared with the OA group (P<0.05). There is no statistical significance in each mRNA between the control group and the drug group (see Figure 5).
Discussion
OA is a degenerative disease characterized by destruction of cartilage. Earlier studies have shown that inflammatory response and infiltration of inflammatory factors play a crucial role in the pathogenesis of OA. The papain-induced rat OA model has the advantages of high stability, convenient operation, limited time and other strength, a classic model used to evaluate the efficacy of drug treatment (11). Therefore, this model was used to study the anti-inflammatory and anti-OA functions of AST. Papain is a proteolytic enzyme, and its mechanism of causing OA is considered the decomposition of proteoglycan in the cartilage matrix, the removal of mitotic inhibitors on the chondrocyte membrane, and the loss of proteoglycan from the cartilage matrix. The researchers believed that one of the earliest remarkable changes in human OA cartilage is an increase in water and a decrease in proteoglycans (11).
The current theory is that inflammation plays a determinant part in the development of OA (12). New research shows that cartilage, bone, and synovium play a crucial role in OA. Cartilage, bone, and synovium, as buffer tissues, participating in the pain process of OA, are the source of inflammatory mediators (13). Many studies have shown that the down-regulation of inflammatory mediators may have a protective impact on the development and progress of OA. Although some breakthroughs have been made in the pathogenesis of the disease, finding a treatment is still a challenge. Relative to inflammatory joint disease, there is no effective drug treatment for OA (14). Non-steroidal anti-inflammatory drugs (NSAIDs) are widely applied in the clinic, yet it can only temporarily alleviate the symptoms of OA, but with increased risk of myocardial infarction. As a traditional Chinese medicine, AST has been reported to have a positive therapeutic effect on rheumatoid arthritis (15). Previous studies have shown that AST in gout mice can reduce the infiltration of inflammatory cells into the synovium and reduce the erosive damage to cartilage, but its mechanism needs to be further studied. In one study, AST has been proved to effectively inhibit the production of TNF-α, IL-1β, IL-6, and IL-12 (16).
In clinical practice, Astilbe chinensis is applied to treat rheumatic joint pain. Astilbe chinensis contains flavonoids, terpenoids, and other active components, which are often used in the treatment of rheumatoid arthritis, gout, hyperuricemia, glomerulonephritis, urethral infection, and other diseases (17). Studies have shown that the rhizome extract of Astilbe chinensis can inhibit the activity of specific inflammatory cells through immunomodulatory therapy, but its mechanism is still unclear. Asparagus glycoside is a dihydroflavonol derivative isolated from the rhizome of Astilbe chinensis, which has various pharmacological effects, including anti-oxidation, anti-inflammation, anti-diabetes, and anti-nephropathy (18,19). Evidence suggests AST selectively suppresses lymphocyte function to reduce dysfunction in arthritis (20) and relieves contact allergies (21). Furthermore, AST alleviates lupus erythematosus symptoms by inhibiting activated immune cells (22). Although it has been reported AST extracted from Poria CocosAstilbe chinensis can inhibit inflammatory factors and reduce immune response, but there is no report on the application of AST in OA (15). Thus, the anti-inflammatory mechanism of AST in the treatment of OA still requires illustration. The study is the first to report using AST in the treatment of OA.
Previous studies have shown that pro-inflammatory factors, including iNOS and COX2, can aggravate cartilage matrix damage and induce chondrocyte apoptosis (23,24). Moreover, elevated iNOS and COX2 can further promote the increase of inflammatory mediators, including NO and PGE2 (25,26), the NF-κB signal pathway has been proved to be related to the pathogenesis of OA (27,28). In the resting state, NF-κB binds to the profilin IκBα to be preserved into the cytoplasm. When stimulated by inflammatory factors like IL-1β, IκBα is phosphorylated and degraded, followed by the entering of P65 translocation into the nucleus. In the nucleus, p65 takes part in the decomposition of metabolic enzymes and the secretion of cytokines and inflammatory mediators. PGE2 is an inflammatory mediator produced by endogenous arachidonic acid-induced by COX-2 through IL-1β, which stimulates the expression of MMP and ADAMTS5 as well. As a subgroup of the member of collagenase, MMP-13 is one of the MMP that is most related to cartilage catabolism. Moreover, ADAMTS5 is regarded as a vital factor in the cleavage of proteoglycan in the pathogenesis of OA (29). Hence, drugs targeting the inhibition of PGE2 through NF-κB have become an effective treatment program for OA.
In this study, the HE staining suggested AST can protect the articular surface and reduce damage. The results of immunohistochemical staining also proved that AST could suppress the expression of PGE2 and had a superior inhibitory effect on inflammatory factors. According to Western blot analysis, it was found that AST can inhibit the release of inflammatory proteins. At the meantime, in the cartilage tissue of rats in the drug group, the mRNA expression of IL-1β, TNF-α, PEG2, and NF-κB was down-regulated, indicating that the inflammatory response was inhibited, and the inhibition of the inflammatory pathway provided a favorable environment for the repairing of articular cartilage, which played a regulatory and preventive role in OA, delayed cartilage degeneration and promoted cartilage repair.
Our results showed that AST significantly inhibits PEGE2 through the NF-κB pathway and plays a critical role in the development of OA. Although the study supplies some experimental data and theoretical basis and expounds the mechanism of traditional Chinese medicine monomer in the treatment of OA. However, there are still some limitations in the research, and further exploration of the synergism between TLR, MAPK, PI3K/AKT, and other inflammatory factors from the relevant molecular pathways is demanded, which is also what the next step of the research will focus.
Acknowledgments
Funding: Science and technology cooperation program of the Department of Science and Technology of Guizhou Province and the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Fund No: LH[2017] No. 7142.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4485
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4485
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4485). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The mice are in line with the specific pathogen-free (SPF) conditions of the Animal Care and Use Committee of Guiyang College of Traditional Chinese Medicine. All the experiments conducted in this study are approved by the experimental animal ethics committee of Guangdong Medical Experimental Animal Center (B201803-4).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137-62. [Crossref] [PubMed]
- DeRogatis M, Anis HK, Sodhi N, et al. Non-operative treatment options for knee osteoarthritis. Ann Transl Med 2019;7:S245. [Crossref] [PubMed]
- Wang M, Shen J, Jin H, et al. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci 2011;1240:61-69. [Crossref] [PubMed]
- Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage 2006;14:367-76. [Crossref] [PubMed]
- Grant A, Amadesi S, Bunnett NW. Protease-Activated Receptors: Mechanisms by Which Proteases Sensitize TRPV Channels to Induce Neurogenic Inflammation and Pain. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007:Chapter 31.
- Wang J, Zhao Y, Xu Q. Astilbin prevents concanavalin A-induced liver injury by reducing TNF-alpha production and T lymphocytes adhesion. J. Pharm. Pharmacol. 2004;56:495-502. [Crossref] [PubMed]
- Yu L, Huang H, Yu LL, et al. Utility of hesperidinase for food function research: enzymatic digestion of botanical extracts alters cellular antioxidant capacities and anti-inflammatory properties. J Agric Food Chem 2014;62:8640-47. [Crossref] [PubMed]
- Li GS, Jiang WL, Yue XD, et al. Effect of astilbin on experimental diabetic nephropathy in vivo and in vitro. Planta Med. 2009;75:1470-75. [Crossref] [PubMed]
- Huang H, Cheng Z, Shi H, et al. Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J Agric Food Chem 2011;59:4562-69. [Crossref] [PubMed]
- Dong L, Zhu J, Du H, et al. Astilbin from Smilax glabra Roxb. Attenuates Inflammatory Responses in Complete Freund's Adjuvant-Induced Arthritis Rats. Evid Based Complement Alternat Med 2017;2017:8246420.
- Han GY, Ling PX, Wang FS, et al. Comparison study on knee osteoarthritis in rabbits induced by different concentrations of papain. Zhongguo Gu Shang 2012;25:424-29. [PubMed]
- Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 2006;14:839-48. [Crossref] [PubMed]
- Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr. Rheumatol. Rep. 2009;11:357-64. [Crossref] [PubMed]
- Bamborough P, Morse MA, Ray KP. Targeting IKKbeta for the treatment of rheumatoid arthritis. Drug News Perspect 2010;23:483-90. [Crossref] [PubMed]
- Dong L, Zhu J, Du H, et al. Astilbin from Smilax glabra Roxb. Attenuates Inflammatory Responses in Complete Freund's Adjuvant-Induced Arthritis Rats. Evid Based Complement Alternat Med 2017;2017:8246420.
- Liang G, Nie Y, Chang Y, et al. Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate- and monosodium urate-induced hyperuricemia and gout in mice. Phytomedicine 2019;59:152772. [Crossref] [PubMed]
- Wang M, Yang XB, Zhao JW, et al. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr Polym 2017;156:390-402. [Crossref] [PubMed]
- Lu CL, Zhu YF, Hu MM, et al. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules 2015;20:625-44. [Crossref] [PubMed]
- Yan R, Xu Q. Astilbin selectively facilitates the apoptosis of interleukin-2-dependent phytohemagglutinin-activated Jurkat cells. Pharmacol Res 2001;44:135-39. [Crossref] [PubMed]
- Cai Y, Chen T, Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res 2003;52:334-40. [Crossref] [PubMed]
- Fei M, Wu X, Xu Q. Astilbin inhibits contact hypersensitivity through negative cytokine regulation distinct from cyclosporin A. J Allergy Clin Immunol 2005;116:1350-56. [Crossref] [PubMed]
- Guo L, Liu W, Lu T, et al. Decrease of Functional Activated T and B Cells and Treatment of Glomerulonephitis in Lupus-Prone Mice Using a Natural Flavonoid Astilbin. PLoS One 2015;10:e0124002. [Crossref] [PubMed]
- Cao M, Westerhausen-Larson A, Niyibizi C, et al. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J 1997;324:305-10. [Crossref] [PubMed]
- Takada K, Hirose J, Yamabe S, et al. Endoplasmic reticulum stress mediates nitric oxide-induced chondrocyte apoptosis. Biomed Rep 2013;1:315-19. [Crossref] [PubMed]
- Dumond H, Presle N, Pottie P, et al. Site specific changes in gene expression and cartilage metabolism during early experimental osteoarthritis. Osteoarthritis Cartilage 2004;12:284-95. [Crossref] [PubMed]
- Ma Y, Tu C, Liu W, et al. Isorhapontigenin Suppresses Interleukin-1beta-Induced Inflammation and Cartilage Matrix Damage in Rat Chondrocytes. Inflammation 2019;42:2278-85. [Crossref] [PubMed]
- Marcu KB, Otero M, Olivotto E, et al. NF-kappaB signaling: multiple angles to target OA. Curr. Drug Targets 2010;11:599-613. [Crossref] [PubMed]
- Kumar A, Takada Y, Boriek AM, et al. Nuclear factor-kappaB: its role in health and disease. J Mol Med (Berl) 2004;82:434-48. [Crossref] [PubMed]
- Hardy MM, Seibert K, Manning PT, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum 2002;46:1789-803. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


