
The protective effect of polyphyllin I on myocardial ischemia/reperfusion injury in rats
Introduction
Currently, acute myocardial infarction (AMI) is a common cardiovascular disease, which leads to high morbidity and mortality around the world. For patients with AMI, the most prompt and effective treatment is to improve myocardial ischemic and restrict the size of myocardial infarction. As far as we know, myocardial reperfusion is so far the principal or only strategy for the treatment of AMI, which is clinically achieved by angioplasty or thrombolytic therapy, thereby promptly restoring blood supply (1). However, this sudden reperfusion should result in secondary cascade damages, called ischemia/reperfusion (I/R) injury, which paradoxically deteriorated ischemic damages and further swelled infarct size (2). Also, myocardial I/R injury could trigger a large variety of pathological changes, including local acute inflammatory reactions, metabolic disorders, and cell apoptosis or necrosis, which ultimately led to cardiac dysfunction (3). Simultaneously, the pathogenesis of myocardial I/R injury is involved. It involves multiple molecular processes, such as reactive oxygen species (ROS) accumulation (4), calcium ion overload (5), inflammatory cytokines influx (6), and mitochondrial permeability transition pore (mPTP) openings (7), among many others. Although there are many necessary research reports on myocardial I/R injury, the clinical effects are not satisfactory. We still need further research to find more effective clinical treatments.
Chinese herbal medicine has a history of thousands of years in the treatment of multiple diseases. Polyphyllin I (PPI), also called Chong Lou saponin I, a steroidal saponin, is a bioactive substance separated from the rhizome of Paris polyphylla. Previous reports revealed that PPI possessed an anti-cancer effect in a variety of cancers through inhibiting the proliferation and growth of tumor cells (8-12), thereby being used as a possible anti-cancer drug candidate. Besides, Shi et al. found that PPI could promote the occurrence of protective autophagy in HCC cells (13). Yang et al. reported that PPI inhibited cell survival capability and eased apoptosis in gefitinib-resistant NSCLC cells and xenograft models (14).
Furthermore, PPI could permeabilize the membrane of human red blood cells (RBC), resulting in increased Ca2+ concentration (15), and PPI also directly triggered mitochondrial swelling (16), thus causing cell apoptosis. Previously, PPI was reported to down-regulate the constitutive phosphorylation of NF-κB p65 protein and its downstream target genes expression in hepatocellular carcinoma (17). Similarly, recent studies revealed that PPI also protects against myocardial I/R injury, remarkably inhibiting the secretion of inflammatory cytokines and suppressing the translocation of p65 (18,19). At present, many drugs have been demonstrated to be effective against reducing myocardial I/R injury in animal models but not involved in clinical practice (1,20,21), and the role of PPI in myocardial I/R injury is still ambiguous.
In this study, we established a model of myocardial I/R in rats, the aim was to investigate the protective effect of PPI on myocardial injury, and also explore the role of PPI on I/R-induced myocardial inflammation and oxidative stress, enriching its pharmacological actions. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3371).
Methods
Primary materials
PPI powder (molecular formula: C44H70O16, molecular weight: 855.017, density: 1.4±0.1 g/cm3, purity ≥98%) was obtained from Sichuan Weikeqi Biological Technology Co., Ltd. (Chengdu, China). Male Sprague-Dawley (SD) rats (≥6 weeks old, 251–300 g) were bought from Zhejiang Chinese medical university Laboratory Animal Research Center (Zhejiang, China).
Primary assay and methods
Animals administration
All male SD rats were placed in the same environment (23±2 °C, relative humidity 55%±5%, and a 12 h light/dark cycle), kept food and water-free. The rats were randomly grouped as follows (n=9): sham group, I/R group, I/R + PPI (50 mg/kg) group, and I/R + PPI (100 mg/kg) group, I/R + PPI (150 mg/kg) group. The sham operation group did not have I/R but was given normal perfusion at the same time. Rats in the sham group and I/R group were given 0.9% physiological saline, yet in I/R + PPI treatment groups were given different doses of PPI (22) by intraperitoneal administration (once a day for 14 days), followed by I/R surgery.
Animal model of myocardial I/R
Besides, all the rats were fasting for 12 h before the operation, then they were anesthetized with 10% chloral hydrate and fastened on the operating table, yet the I/R procedures were achieved referring to previous descriptions (23). Briefly, open the left chest to reveal the heart, peel the pericardium to find the coronary artery, and ligate the left anterior descending coronary (LAD). LAD ligation induced myocardial ischemia for 30 minutes and reperfusion for 120 minutes—and the same surgery for sham operation without LAD ligation. Twenty-four hours after I/R surgery, cardiac tissues, or blood samples were collected and stored (−80 °C) for further analysis.
All animal experiment steps were conducted following the National Institutes of Health (NIH) guide for the care and use of laboratory animals [8th edition, National Academies Press (US), 2011] and approved by Chengdu University of Traditional Chinese Medicine.
Echocardiography
SD rats were narcotized by intraperitoneal injection of 2% isoflurane and placed in a flat position. The echocardiography measurement of left ventricular function was done by digital ultrasound equipment Vevo 2100 (VisualSonics, Ontario, Canada) and 18 MHz transducers after cardiac perfusion. Then, M-mode recordings of the left ventricular ejection fraction (LVEF), left ventricular wall thickness (LVWT), and left ventricular end-systolic volume (LVESV) were calculated. All the results were averaged from at least three consecutive cardiac cycles measuring from the M-mode images and were analyzed by a blinded researcher.
Histopathological analysis
Rat heart tissues were excised and fixed in 10% formalin buffer. After paraffin embedding, the tissues sections were chipped to 4 µm thickness and then stained with hematoxylin-eosin (HE) liquid for the histopathological examination.
Immunohistochemistry (IHC) assay
The rat heart tissues were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at a thickness of 4 µm according to standard procedures. Next, deparaffinized sections were then hydrated. Furthermore, endogenous peroxidase activity was blocked by Hydrogen Peroxide Block for 15 min. After antigen retrieval in 10 mM heated citrate buffer for 10 min, then the sections were incubated with primary antibody: anti-anti-Caspase-3 antibody (ab4051, 1:500, Abcam, UK) overnight at 4 °C. Subsequently, the corresponding secondary antibody was incubated for 1 h at room temperature. Finally, the specimens were stained with DAB kit (Boster Biological Technology Co., Ltd., Wuhan, China). Then, the specimens were washed with PBS, and images were captured under confocal microscopy (Leica, Wetzlar, Germany).
TUNEL staining
Rats were euthanized, and paraffin sections were prepared as above method. TUNEL assay was conducted using a TUNEL kit (R&D Systems, Minnesota, USA), according to the manufacturer’s protocols. Five fields of vision were randomly selected under an optical microscope (Leica, Wetzlar, Germany), positive brown cells and total cells were counted, and the apoptosis rate was calculated.
Determination of inflammatory cytokines
Blood samples were centrifuged for 10 min at 2,000 G, and supernatants were collected, the serum levels of TNF-α, IL-6, iNOS, and IL-10 were detected using ELISA kits, according to the manufacturer‘s protocols (Thermo Fisher, Waltham, MA, USA). The results at 450 nm was recorded and the sample concentration calculate was calculated with the best fitting curve for data analysis.
Oxidative stress detection
After I/R, blood samples were collected from the left common carotid artery and centrifuged for 10 min at 2,000 G, the levels of SOD, ROS, MDA, and GSH in the serum were measured with professional test kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and all operating procedures were performed according to the manufacturer’s instructions.
Determination of cardiac injure biomarkers
The myocardial damage was evaluated by measuring the level of myocardial-specific markers, including AST, CK-MB, cTnT and LDH. myocardial perfusion injury is accompanied by changes in the content of some biomarkers. Therefore, changes in serum levels of biomarkers can reflect tissue damage. according to the manufacturer’s instructions, we tested the expression of cTnI, CK-MB, Mb and LDH according to the instructions provided in the purchased kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Western blot (WB) analysis
The total protein samples were extracted with RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). Protein lysates were quantified using a Pierce™ BCA protein assay kit (23227, Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s protocols. About 50 µg of proteins loaded onto 12% SDS PAGE gels, All membranes were blocked using 5% nonfat dry milk in Tris-buffered saline (TBS) at 4 °C for 30 min, and were incubated with the following primary antibodies: anti-Ki67 antibody (ab16667, 1:500, Abcam, UK), anti-Surviving antibody (ab134170, 1:500, Abcam, UK), anti-Bax antibody (ab32503, 1:500, Abcam, UK), and anti-Bcl-2 antibody (ab196495, 1:500, Abcam, UK), anti-NF-κBp65 antibody (ab16502, 1:500, Abcam, UK) and anti-NF-κBp65 (phosphor S536) antibody (ab86299, 1:500, Abcam, UK). Next, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody, respectively. Finally, the bands were exposed to ECL reagent (Thermo Fisher, Waltham, MA, USA). β-actin was used as the loading control.
Statistical analysis
All the experiments were conducted at least three times, and the data were expressed as mean ± SD. Statistical analysis was conducted using IBM SPSS Statistics 25.0. (IBM, Armonk, USA). Student’s t-test was used to evaluate the difference between the two groups, and one-way ANOVA was used to analyze the significance of the difference among different treatment groups. P<0.05 showed statistical significance.
Results
PPI alleviated pathological injury of cardiac tissue and prevented the cardiac function
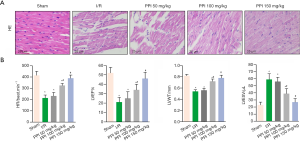
The myocardial sections were stained via HE staining to assess the myocardial injury. As showed in Figure 1A, the sham group displayed no apparent difference in the morphological structures, whereas the I/R group showed obvious myocardial fiber fracture, cellular edema, necrosis, and inflammatory infiltration. Interestingly, different doses of PPI (50, 100, 150 mg/kg) significantly attenuated myocardial tissue injury in the myocardium compared with I/R group. Following, we used echocardiography analysis to investigate cardiac function during acute myocardial I/R injury. As showed in Figure 1B, I/R treatment significantly decreased HR, LVEF, and LVWT, accompanied by increased LVESV, respectively. However, PPI remarkably improved the cardiac function compared with the I/R group.
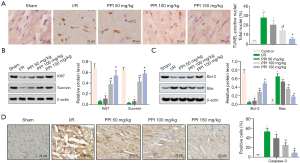
PPI attenuated apoptosis after I/R
TUNEL staining demonstrated the anti-apoptotic effect of PPI (50, 100, 150 mg/kg) in myocardial I/R rats (Figure 2A). Parallelly, the protein levels of Ki67 and Surviving were down-regulated in the I/R group (Figure 2B). As expected, PPI reversed the change, compared with I/R group. Furthermore, we evaluated the expression of Bcl-2, Bax, and caspase-3 proteins by WB and IHC assay, respectively. According to our results, the levels of caspase-3 and Bax were up-regulated, the level of Bcl-2 was down-regulated by I/R. Contrarily, PPI significantly down-regulated the levels of caspase-3 and Bax, while up-regulated the level of Bcl-2, compared with I/R group (Figure 2C,D).
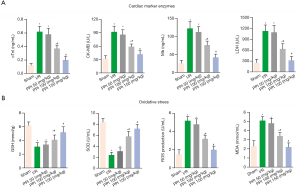
PPI reduced myocardial injury and oxidative stress in rats
To further evaluate IR-induced myocardial injury, we measured the release of several biomarkers (cTnI, CK-MB, Mb, and LDH) in serum. Figure 3A showed the expression of cTnI, CK-MB, Mb, and LDH were increased in I/R group, compared with sham group. On the contrary, PPI (50, 100, 150 mg/kg) reversed the results of I/R processing. Next, we investigated the serum levels of oxidative stress makers. Figure 3B showed the expression of SOD and GSH were markedly increased, and the expression of ROS and MDA were decreased after I/R. PPI significantly increased antioxidant levels of SOD and GSH and decreased oxidant levels of ROS and MDA in rats with myocardial I/R injury, compared with I/R group.
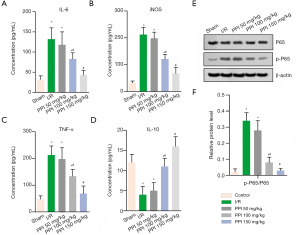
PPI inhibited I/R-induced inflammatory response and impaired the activity of NF-κB p65
To determine the role of PPI in inflammation, we evaluated the serum levels of TNF-α, IL-6, iNOS, and IL-10 via ELISA assay. As showed in Figure 4, I/R remarkably up-regulated the levels of TNF-α, IL-6, iNOS while down-regulated IL-10. On the contrary, the protein levels of TNF-α, IL-6, and iNOS were effectively up-regulation, and IL-10 was down-regulation in the PPI groups (50, 100, 150 mg/kg), compared with I/R group (Figure 4A,B,C,D). Therefore, these data showed that PPI could effectively attenuate I/R-induced inflammation. Moreover, WB analysis showed that p-p65/p65 dramatically lifted in the IR group, and treatment with PPI inverted the ratio of p-P65/P65 (Figure 4E,F).
Discussion
Studies show that I/R injury induces free radical formation and inflammation within hours, leading to excessive production of oxidants and inflammatory mediators (24). Oxidative stress and inflammation are vital processes in the pathogenesis of myocardial I/R injury (25). In the present study, PPI significantly increased the levels of SOD and GSH (antioxidant makers) and decreased levels of ROS, and MDA (oxidant makers). Besides, myocardial I/R causes cytokines such as TNF-α, IL-6, and IL-1β. In this, as an important cytokine in inflammation, IL-6 plays an important role in I/R induced injury, which is a downstream target of IL-1β. TNF-α secreted by macrophages promotes inflammatory cascade by increasing the release of other proinflammatory cytokines and influencing the recruitment of neutrophils (26). Basing on these findings, we found that PPI significantly decreased the expression of TNF-α, IL-6, and iNOS, increased IL-10 expression. These results, together, were consistent with the pathological evaluation of myocardial tissues.
Ischemia triggers apoptosis of cardiomyocytes and then expands and partially contributes to cardiac death through reperfusion (27). Song et al. reported that I/R injury markedly induced activation of 12/15-LOX and resulted in cardiomyocyte apoptosis, increased caspase-3 activity, and Bax/Bcl-2 ratio (28). Especially, cleaved caspase-3 was a crucial triggering factor (29), which activated various downstream proteases, such as cysteine protease, inhibiting cell proliferation, and inducing apoptosis. In our study, TUNEL staining and the detection of Caspase-3, which were the apoptosis markers, revealing the number of apoptotic cells were decreased and the expression of caspase-3 was down-regulated by PPI treatment. Besides, the expression of Bcl-2 was dereased and Bax was increased. Interestingly, we also found PPI decreased the levels of injury bio-markers (cTnI, CK-MB, Mb, and LDH) in serum, and up-regulated Ki67 and Surviving protein levels, suggesting PPI attenuated myocardial injury via mediating myocardial apoptosis in rats.
NF-κB pathways take part in many physiological functions, including hypertension, heart failure, and myocardial hypertrophy. NF-κB, as a redox-sensitive transcription factor, is usually present in the cytoplasm as a heterodimer. The prototypical signals, such as LPS, IFN-γ, IL-1, and TNF-α, could induce phosphorylation of I-κB protein by upstream kinases (IKK), which resulted in the ubiquitination and degradation of I-κB, thereby releasing the NF-κB and further activating NF-κB (19). Many studies showed that NF-κB is activated after myocardial I/R injury (20,30). Therefore, inactivation of the NF-κB pathway in cardiomyocytes has cardioprotective effects against I/R-induced myocardial oxidative stress injury, cell death, and other injuries (31). Our study showed PPI inhibited the activity of NF-κB p65 (22). Ulteriorly, active NF-kB then translocates into the nucleus and activates downstream target genes, which experience ROS-mediated activation, stimulating the transcription of several protein mediators, for example, proinflammatory cytokines and pro-fibrotic response that activate several cell death pathways (32). Our results showed that PPI protects myocardial injury mediated NF-κB pathway by inhibiting the inflammatory response and oxidative stress contradicting these reports. Nevertheless, this is still to be investigated in the future.
Conclusively, this study demonstrated PPI alleviated myocardial I/R injury, inhibited myocardial apoptosis, and weakened the inflammation response and oxidative stress following I/R. Also, PPI mediated the activity of NF-κB p65. Hence, the findings suggested PPI existed a protective role of myocardial I/R injury in rats, and it supplied a new vision for I/R treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3371
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3371
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3371). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee of Chengdu University of Traditional Chinese Medicine approved all animal experiments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vincent A, Covinhes A, Barrère C, et al. Acute and long-term cardioprotective effects of the Traditional Chinese Medicine MLC901 against myocardial ischemia-reperfusion injury in mice. Sci Rep 2017;7:14701. [Crossref] [PubMed]
- Pælestik KB, Jespersen NR, Jensen RV, et al. Effects of hypoglycemia on myocardial susceptibility to ischemia-reperfusion injury and preconditioning in hearts from rats with and without type 2 diabetes. Cardiovasc Diabetol 2017;16:148. [Crossref] [PubMed]
- Kitano K, Usui S, Ootsuji H, et al. Rho-kinase activation in leukocytes plays a pivotal role in myocardial ischemia/reperfusion injury. PLoS One 2014;9. [Crossref] [PubMed]
- Ravindran S, Boovarahan SR, Shanmugam K, et al. Sodium thiosulfate preconditioning ameliorates ischemia/reperfusion injury in rat hearts via reduction of oxidative stress and apoptosis. Cardiovasc Drugs Ther 2017;31:511-24. [Crossref] [PubMed]
- Mozaffari MS, Liu JY, Abebe W, et al. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis 2013;3:180-96. [PubMed]
- Badalzadeh R, Baradaran B, Alihemmati A, et al. Troxerutin preconditioning and ischemic postconditioning modulate inflammatory response after myocardial ischemia/reperfusion injury in rat model. Inflammation 2017;40:136-43. [Crossref] [PubMed]
- Xu T, Ding W, Ao X, et al. ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol 2019;20:414-26. [Crossref] [PubMed]
- Dong R, Guo J, Zhang Z, et al. Polyphyllin I inhibits gastric cancer cell proliferation by downregulating the expression of fibroblast activation protein alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated fibroblasts. Biochem Biophys Res Commun 2018;497:1129-34. [Crossref] [PubMed]
- Zhang Y, Huang P, Liu X, et al. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol Sci 2018;137:305-12. [Crossref] [PubMed]
- Xiang S, Zou P, Tang Q, et al. HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration- resistant prostate cancer cells in vitro and in vivo. Biochim Biophys Acta Gen Subj 2018;1862:589-99. [Crossref] [PubMed]
- Gu L, Feng J, Zheng Z, et al. Polyphyllin I inhibits the growth of ovarian cancer cells in nude mice. Oncol Lett 2016;12:4969-74. [Crossref] [PubMed]
- Kong M, Fan J, Dong A, et al. Effects of polyphyllin I on growth inhibition of human non-small lung cancer cells and in xenograft. Acta Biochim Biophys Sin 2010;42:827-33. [Crossref] [PubMed]
- Shi YM, Yang L, Geng YD, et al. Polyphyllin I induced-apoptosis is enhanced by inhibition of autophagy in human hepatocellular carcinoma cells. Phytomedicine 2015;22:1139-49. [Crossref] [PubMed]
- Yang Q, Chen W, Xu Y, et al. Polyphyllin I modulates MALAT1/STAT3 signaling to induce apoptosis in gefitinib-resistant non-small cell lung cancer. Toxicol Appl Pharmacol 2018;356:1-7. [Crossref] [PubMed]
- Gao M, Cheung KL, Lau IP, et al. Polyphyllin D induces apoptosis in human erythrocytes through Ca2+ rise and membrane permeabilization. Arch Toxicol 2012;86:741-52. [Crossref] [PubMed]
- Ong RCY, Lei J, Lee RKY, et al. Polyphyllin D induces mitochondrial fragmentation and acts directly on the mitochondria to induce apoptosis in drug-resistant HepG2 cells. Cancer Letters 2008;261:158-64. [Crossref] [PubMed]
- Han W, Hou GX, Liu L, Polyphyllin I. PPI) increased the sensitivity of hepatocellular carcinoma HepG2 cells to chemotherapy. Int J Clin Exp Med 2015;8:20664-9. [PubMed]
- Zhu T, Wu WJ, Yang SY, et al. Polyphyllin I Inhibits Propionibacterium acnes-Induced Inflammation In Vitro. Inflammation 2019;42:35-44. [Crossref] [PubMed]
- Wang Q, Zhou X, Zhao Y, et al. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Front Immunol 2018;9:2091. [Crossref] [PubMed]
- Liu X, Wang Y, Zhang M, et al. Ticagrelor reduces ischemia- reperfusion injury through the NF-κB-dependent pathway in rats. J Cardiovasc Pharmacol 2019;74:13-9. [Crossref] [PubMed]
- Wu J, Yang Y, Xun N, et al. Osthole attenuates myocardial ischemia/reperfusion injury in rats by inhibiting apoptosis and inflammation. Am J Transl Res 2018;10:1109-16. [PubMed]
- Liu Y, Yuan YJ, Wu Y, et al. Polyphyllin I alleviates myocardial ischemia/reperfusion injury via nuclear factor-κB signal pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019;31:746-9. [PubMed]
- Liu K, Wang F, Wang S, et al. Mangiferin attenuates myocardial ischemia- reperfusion injury via MAPK/Nrf-2/HO-1/NF-κB in vitro and in vivo. Oxid Med Cell Longev 2019;2019:7285434. [Crossref] [PubMed]
- Zhang HR, Bai H, Yang E, et al. Effect of moxibustion preconditioning on autophagy-related proteins in rats with myocardial ischemia reperfusion injury. Ann Transl Med 2019;7:559. [Crossref] [PubMed]
- Al-Salam S, Hashmi S. Myocardial Ischemia Reperfusion Injury: Apoptotic, Inflammatory and Oxidative Stress Role of Galectin-3. Cell Physiol Biochem 2018;50:1123-39. [Crossref] [PubMed]
- Cai Z, Liu J, Bian H, et al. Suppression of P2X7/NF-kappaB pathways by Schisandrin B contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Arch Pharm Res 2016;39:499-507. [Crossref] [PubMed]
- Tan H, Chen L, Ma J. Penehyclidine hydrochloride post-conditioning reduces ischemia/reperfusion-induced cardiomyocyte apoptosis in rats. Exp Ther Med 2017;14:4272-8. [PubMed]
- Song L, Yang H, Wang HX, et al. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis 2014;19:567-80. [Crossref] [PubMed]
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999;68:383-424. [Crossref] [PubMed]
- Gray CB, Suetomi T, Xiang S, et al. CaMKIIδ subtypes differentially regulate infarct formation following ex vivo myocardial ischemia/reperfusion through NF-κB and TNF-α. J Mol Cell Cardiol 2017;103:48-55. [Crossref] [PubMed]
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res 2011;108:1122-32. [Crossref] [PubMed]
- Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 2000;59:13-23. [Crossref] [PubMed]


