Therapeutic effects and safety of early use of sacubitril/valsartan after acute myocardial infarction: a systematic review and meta-analysis
Introduction
Acute myocardial infarction (AMI) refers to myocardial necrosis caused by acute and persistent ischemia and hypoxia of the coronary artery (1-3). Acute and persistent pain behind the sternum is common in clinical settings, and rest and nitrate drugs cannot alleviate this symptom completely. Additionally, this symptom is accompanied by an increase in the activity of serum myocardial enzymes, progressive changes in electrocardiograms, arrhythmia, shock, or cardiac failure, which usually pose a threat to life. AMI occurs in Europe and America most frequently. In the United States, about 1,500,000 people suffer from myocardial infraction every year. The incidence of this disease used to be low in China, but a growing trend in the incidence of AMI has been observed in recent years (4,5).
Percutaneous coronary intervention (PCI) is an effective therapeutic method for AMI because it can remove coronary artery stenosis, relieve chest pain and other clinical symptoms, and reduce mortality (6-8). However, the incidence of postoperative complications is high, and such complications result in a significant increase in the reconstruction rate of target lesion blood transport, the formation rate of stent thrombosis, and mortality. Among all the postoperative complications, cardiac dysfunction is one of the most common postoperative complications, and it affects the quality of life and physical health of patients. The main pathological changes lie in the ventricular remodeling caused by the excessive activation of the sympathetic nerve and renin-angiotensin-aldosterone system (RAAS) (9,10). At present, the use of conventional anti-cardiac failure drugs after AMI reduces the mortality of patients to a large extent, but the incidence of cardiac dysfunction in postoperative complications remains high. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) have been shown to improve the prognosis of patients (11-14). ACEIs not only enhance the activity of angiotensin-converting enzymes, but can also alleviate the cardiac load of patients, reduce myocardial reperfusion injuries, inhibit ventricular remodeling, and improve cardiac function.
Sacubitril/valsartan is a type of angiotensin receptor-neprilysin inhibitor (ARNI). It is helpful in generating early myocardial vessels after AMI, inhibiting ventricular remodeling, mitigating cardiac failure, and improving patients’ prognosis by inhibiting active enkephalin enzymes and the angiotensin II receptor of RAAS. According to Vaduganathan et al. [2020] (15), the use of sacubitril/valsartan sodium tablets in treating patients with AMI reduces the hospitalization rate of patients with cardiac failure and the mortality of patients with cardiovascular diseases more than enalapril. A large number of clinical trials have been successively carried out to investigate the clinical curative effects of the early adoption of sacubitril/valsartan after AMI, and some positive effects have been observed. However, there is no consensus on its effect in clinical treatment.
A systematic evaluation refers to the comprehensive retrieval of all relevant research articles worldwide, the strict assessment of each article included in the study, the comprehensive analysis and evaluation of the results of all the combined research, and the generation of comprehensive conclusions at the end. It aims to reduce the risk of bias as much as possible to provide scientific medical evidence. A meta-analysis refers to a statistical method of combining multiple studies of the same type with different results into a quantitative indicator. The innovation of this research is that we conducted a comprehensive retrieval, systematic evaluation, and meta-analysis of randomized controlled trials (RCTs) examining the use of sacubitril/valsartan in AMI patients to provide scientific medical evidence for the clinical adoption of sacubitril/valsartan in the treatment of AMI. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-210/rc).
Methods
Inclusion and exclusion criteria
Articles were included in the research if they met the following inclusion criteria: (I) an RCT that investigated the treatment of patients with AMI by sacubitril/valsartan sodium tablets; (II) the AMI patients met the relevant standards of the “Management Guide of Patients with Acute Coronary Syndrome with Unsustained ST-segment Elevation” published by the European Society of Cardiology in 2020 and the “Management Guide of Non-ST-Segment Elevation AMI” published by European Society of Cardiology in 2020 (16,17); (III) the patients in the control group received conventional treatment of ACEI/ARB and those in the experimental group received sacubitril/valsartan sodium tablets (20–200 mg each time, twice per day; and (IV) the main result indicators included adverse cardiovascular events, the rehospitalization rate, and the mortality rate, and the secondary result indicators included low blood pressure and left ventricular ejection fraction (LVEF).
Articles were excluded from the research if they met the following inclusion criteria: (I) the article was a meeting, report, or abstract for which the whole-text information was unavailable; (II) the research objects were animals; (III) the research objects were women who were pregnant or in the breast-feeding period; (IV) the patients had malignant tumors; and/or (V) the data were incomplete or the original data were unavailable.
Article retrieval
English databases, including American National Library of Medicine, Medline, and Embase, were searched by computer. Chinese databases, including Chinese Biomedical Literature Database, Chinese National Knowledge Infrastructure (CNKI), Wanfang, VIP, and Google Scholar, were also searched by computer. The retrieval period ran from the establishment of the databases to July 20, 2021. The English databases were searched using combinations of the following terms: AMI, ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), sacubitril/valsartan, and angiotensin receptor enkephalinase inhibitors. The Chinese databases were searched using combinations of the following terms: AMI, acute STEMI, acute NSTEMI, sacubitril/valsartan sodium tablets, and angiotensin receptor enkephalinase inhibitors. The above terms were combined freely, and the articles were then selected after multiple searches. The selected articles were further traced by search engines to obtain updates on the progress of the research and collect the data.
Data extraction
The data were assessed by 2 researchers according to the above-mentioned criteria, and the data were entered into a unified Excel worksheet. The data retrieved and included in the research included the research title, the first author and publication year, general data about the research objects (age and gender), the source of the research objects, the sample size, the main result indicators (adverse cardiovascular events, rehospitalization rate, and mortality), and secondary result indicators (hypoglycemia and LVEF).
Quality evaluation and bias risk assessment
The Cochrane Handbook 5.0 risk assessment table were adopted to determine the quality of the included articles. The following 5 aspects were considered: the use of the random assignment method in the random sequence, the use of the blinding method, the use of allocation concealment, the integrity of the data results, and the research results. All 5 aspects were rated as “high-risk bias”, “low-risk bias”, or “unclear”. The final evaluation is low quality, medium quality, and high-quality research. The risk of bias was assessed by 2 professionals simultaneously, and any disagreement was resolved by discussion.
Statistical analysis
StataSE12.0 software was used for the statistical analysis. The risk of bias of the included references was assessed using the risk of bias assessment chart of Rev Man 5.3 software. The enumeration data are presented as the relative risk (RR) and 95% confidence interval (CI). The measurement data are presented as the mean difference (MD) and 95% CI. The heterogeneity among the results of all the studies was assessed by the χ2 test and I2 test. When P>0.01 and I2<50%, the fixed-effects model was used in the meta-analysis. When P<0.01 and I2>50%, the random-effects model was used in the meta-analysis. The test results of the combined effects were expressed by the Z value. The P value was derived according to the Z value. When P<0.05, the difference was considered statistically significant.
Results
Retrieval results and basic information about the included articles
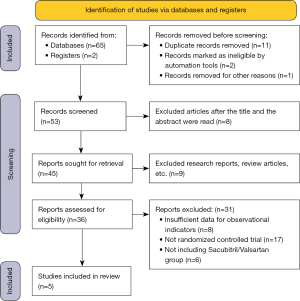
A total of 65 related articles and 2 register website articles were retrieved and obtained by computer. Among these retrieved articles, 11 articles that had been published repeatedly, 2 disqualified articles, and 1 article excluded for other reasons were excluded, and 53 remained. Among the 53 remaining articles, 8 articles were excluded after their titles and abstracts were reviewed, and 45 articles remained. After another 9 articles were excluded, including literature reviews, summaries, and meeting reports, 36 articles remained. Eight other articles were excluded, as the necessary observation indicator data were not included in the articles. Additionally, 17 papers in the non-RCT were excluded, and 6 papers in the experimental group that did not use Sacubitril/Valsartan were excluded. Ultimately, 5 articles (18-22) were included in the meta-analysis. Figure 1 shows the retrieval process.
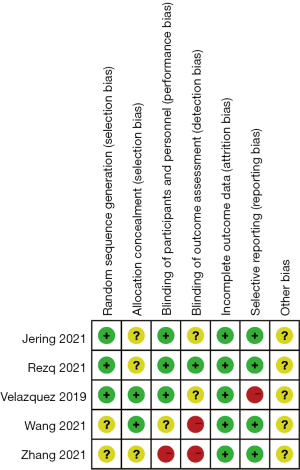
Among the 5 included articles, there were 3,517 patients in the experimental group and 3,518 patients in the control group. The first author, publication year, number of cases, treatment course, and result indicators were described in detail in these articles. Table 1 shows the basic feature data and the data for each result indicator. Among the 5 included articles, 4 articles have scores ranging from 4 to 7, and 1 article had a score ranging from 0 and 3.
Table 1
| First author | Publication year | Sample size | Age, years | Intervention measures | Length of treatment (months) | Result indicators | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Experimental group | Control group | Experimental group | Control group | Experimental group | ||||||
| Jering (18) | 2021 | 2,831 | 2,830 | 64 | 64 | Ramipril (5 mg, 2 times/d) | Sacubitril-valsartan (97–103 mg, 2 times/d) | 23 months | Mortality, rehospitalization rate, and low blood pressure | ||
| Rezq (19) | 2021 | 100 | 100 | 57±11.6 | 52±9.2 | Angiotensin-converting enzyme inhibitor | Sacubitril-valsartan | 6 months | Total incidence of cardiovascular events, rehospitalization rate, and LVEF | ||
| Velazquez (20) | 2019 | 441 | 440 | 63 | 61 | Enalapril | Sacubitril-valsartan | 8 weeks | High blood pressure and low blood pressure | ||
| Wang (21) | 2021 | 69 | 68 | 60.56±7.62 | 59.13±7.15 | Enalapril (2.5–5 mg, 2 times/d) + conventional basic treatment | Sacubitril/valsartan (50–100 mg, 2 times/d) + conventional basic treatment | 6 months | Total incidence of cardiovascular events, rehospitalization rate, mortality, low blood pressure, and LVEF | ||
| Zhang (22) | 2021 | 77 | 79 | 60.0±10.9 | 60.3±11.7 | 6 months | Enalapril + conventional basic treatment | Sacubitril/valsartan + conventional basic treatment | Total incidence of cardiovascular events, rehospitalization rate, and LVEF | ||
LVEF, left ventricular ejection fraction.
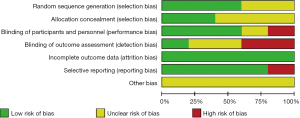
Results of the risk of bias evaluations of the included articles
The risk of bias of the included articles was evaluated using the Cochrane Handbook 5.0, and risk of bias proportion charts were made (see Figures 2,3). All of the 5 articles included in the research were grouped randomly. Two articles used the computerized random number table method, and 1 used the single-blind method. No study used the allocation concealment method. Additionally, incomplete data and selective reporting were not detected. The quality of all the included articles was of the upper-intermediate level.
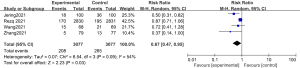
Meta-analysis of the total incidence of adverse cardiovascular events
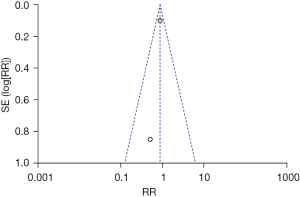
The total incidence of adverse cardiovascular events was compared and studied in 3 of the articles. The results of the meta-analysis of the 3 articles were P=0.41 and I2=0%, which indicated statistical homogeneity. The results of the combined analysis of the fixed-effects model and the meta-analysis were RR =0.61 (95% CI: 0.46, 0.82), and the results of the significance testing were Z=3.36 and P=0.0008, which indicated that the differences were statistically significant (see Figure 4). The results showed that the total incidence of adverse cardiovascular events in the sacubitril/valsartan group was lower than that in the control group.

One article was excluded by the sensitivity analysis because it had no obvious effect on overall heterogeneity. The inverted funnel figure illustrated that the data were concentrated near the central line, and the circles in some of the studies were roughly symmetrical. The results indicated the high accuracy of the research and no publication bias (see Figure 5).
Meta-analysis of mortality
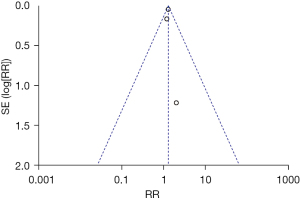
Postoperative mortality was compared and studied in 2 articles. The results of the 2 articles were P=0.52 and I2=0%, which indicated statistical homogeneity. The results of the combined analysis of the fixed-effects model and the meta-analysis were RR =0.87 (95% CI: 0.71, 1.06). The results of the significance testing were Z=1.35 and P=0.18, which indicated that the differences were not statistically significant (see Figure 6). The results showed that the postoperative mortality of patients in the sacubitril/valsartan group did not decrease.
The inverted funnel figure showed that the data were concentrated near the central line, which indicated the high accuracy of the research and no publication bias (see Figure 7).
Meta-analysis of the rehospitalization rate
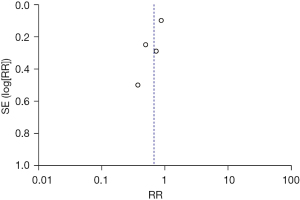
The rehospitalization rate was compared and studied in 4 articles. The results of the meta-analysis of the 4 articles were P=0.09 and I2=54%, which showed statistical heterogeneity. The results of the combined analysis of the random-effects model and the meta-analysis were RR =0.67 (95% CI: 0.47, 0.95), and the results of the significance testing were Z=2.23 and P=0.03, which indicated that the differences were statistically significant (see Figure 8).
Two articles were excluded by the sensitivity analysis because they had no significant effect on the overall heterogeneity. The inverted funnel figure showed that the data were concentrated, and the circles in some studies were roughly symmetrical with the central line, which indicated the high accuracy of the research and no publication bias (see Figure 9).
Meta-analysis of low blood pressure
The low blood pressure data of the 2 groups were compared and studied in 3 articles. The results of the meta-analysis of the 3 articles were P=0.82 and I2=0%, which showed statistical homogeneity. The results of the combined analysis of the fixed-effects model and the meta-analysis were RR =1.28 (95% CI: 1.18, 1.40), and the results of the significance testing were Z=5.58 and P<0.00001, which illustrated that the differences were statistically significant (see Figure 10).
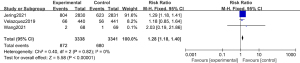
The inverted funnel figure illustrated that the data were concentrated near the central line, and the circles in some studies were roughly symmetrical, which indicated the high accuracy of the research and no publication bias (see Figure 11).
Meta-analysis of LVEF
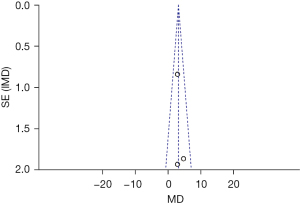
The LVEF data of the 2 groups were compared and studied in 3 articles. The results of the meta-analysis of the 3 articles were P=0.64 and I2=0%, which indicated statistical homogeneity. The results of the combined analysis of the fixed-effects model and the meta-analysis were MD =3.09 (95% CI: 1.69, 4.49), and the results of the significance testing were Z=4.33 and P<0.0001, which demonstrated that the differences were statistically significant (see Figure 12).
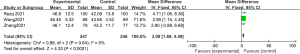
The inverted funnel figure showed that the data were concentrated near the central line, and circles in some studies were roughly symmetrical. The results indicated the high accuracy of the research and no publication bias (see Figure 13).
Discussion
Abnormal ventricular contraction occurs after AMI, and the sympathetic nerve and RAAS are excessively activated, which results in ventricular remodeling. Postoperative cardiac dysfunction is very likely to appear by PCI. In severe cases, pump failure can occur, which can reduce coronary perfusion and aggravate the original myocardial ischemia, or lead to an increase in myocardial oxygen consumption resulting from myocardial stiffness, and further exacerbate progressive cardiac failure (23). At present, the drugs recommended by the “Management Guide of Patients with Acute Coronary Syndrome with Unsustained ST-segment Elevation” still cannot effectively inhibit the excessive activation of neuroendocrine in the pathogenesis of cardiac failure after AMI, and cannot correct hemodynamic disorders in time. As a result, the overall curative effects are not ideal for clinical treatment. Thus, the selection of timely and effective drug treatment after PCI not only promotes the quick recovery of the cardiac function of patients, but also improves the prognosis of patients.
Sacubitril/valsartan plays a positive role in the clinical treatment of chronic cardiac failure. Since 2016, sacubitril/valsartan has been recommended for the treatment of patients with chronic cardiac failure by the “European Chronic Cardiac Failure Guide, the American Cardiac Failure Management Guide, and the Chinese Cardiac Failure Guide” (4). However, no consensus has been reached in the research of the use of sacubitril/valsartan to treat cardiac failure after AMI. Over time, more and more animal experiments and clinical trials have been conducted on the use of sacubitril/valsartan in the treatment of AMI, and breakthroughs have been made. Additionally, the clinical population for whom sacubitril/valsartan is applicable has increased greatly.
Sacubitril/valsartan is a double-effect compound preparation of enkephalinase inhibitors and ARB that functions to affect dual anti-neuroendocrine system activity. Among the components of this preparation, sacubitril is a new molecular entity that blocks the enkephalin enzyme effect and protects the cardiac neurohormonal control system. Additionally, sacubitril reduces the decomposition of bradykinin and natriuretic peptide. It not only promotes the activity of natriuretic peptide to expand blood vessels, excrete sodium, and induce diuresis, it also inhibits profibrotic signaling markers to function in cardiac failure. This inhibition helps to protect the kidneys (24). Valsartan is a kind of common anti-high blood pressure drug, which is used as an inhibitor of the angiotensin II receptor. This drug inhibits the activation of RAAS. It not only prevents the expansion of blood vessels via the angiotensin receptor, it also resists aldosterone to promote diuresis and the excretion of sodium, and reduces the incidence of water-sodium retention (25). The combination of sacubitril and valsartan not only prevents the cardiovascular system from being damaged by enkephalinase inhibitors, it also has a collaborative mechanism by which it expands the blood vessels, excretes sodium, and promotes diuresis to avoid reverse ventricular remodeling, improve hemodynamics, and protect the kidneys.
According to the latest research, the early adoption of sacubitril/valsartan after PCI in the emergency treatment of patients with AMI significantly improves left ventricular remodeling, reduces the incidence of cardiac dysfunction and adverse cardiovascular events during patients’ follow-up visits, and lowers the rehospitalization rate and mortality rate. Based on comprehensive searches and a strict screening, 5 studies, comprising 7,035 patients, were included in this meta-analysis.
The Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor with Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADISE-MI) is a multinational, double-blind, and active controlled trial. In the trial, the research objects who suffered from AMI for 0.5 to 7 days were randomly selected, and treated with sacubitril/valsartan or ramipril. The main outcome of the PARADISE-MI research was reported at the 70th Annual Scientific Meeting of American College of Cardiology, but it has not yet been published in books or journals. The PARADISE-MI qualified for inclusion in the research. The results of this meta-analysis showed the total incidence of adverse cardiovascular events in the sacubitril/valsartan group was significantly lower than that in the control group (RR =0.61 (95% CI: 0.46, 0.82), and the results of the significance testing were Z=3.36 and P=0.0008). Additionally, the rehospitalization rate of the sacubitril/valsartan group was significantly lower that of control group [RR =0.67 (95% CI: 0.47, 0.95), and the results of the significance testing were Z=2.23 and P=0.03]. In terms of LVEF, the level of LVEF in the sacubitril/valsartan group was significantly higher than that in the control group [MD =3.09 (95% CI: 1.69, 4.49)], and the results of the significance testing were Z=4.33 and P<0.0001). The above results were consistent with the outcome of a related analysis conducted by Torrado et al. [2018] (26). The effects of sacubitril/valsartan in patients with left ventricular systolic dysfunction after AMI and hospitalization caused by adverse cardiovascular events and cardiac failure offer a new therapeutic direction for cardiac failure after myocardial infarction.
All of the 5 articles included in the meta-analysis were grouped randomly. Among these articles, 2 used the computerized random number table method, and 1 used the single-blind method. The limitations of this meta-analysis include the overall intermediate level of the quality of the articles and the inadequacy of the sample sizes. Additionally, there were some drawbacks in relation to the statistical methods. For example, the use of specific randomized methods was not mentioned in a number of the articles, and the medication notice for Western conventional drug treatment was not mentioned explicitly in several articles. Additionally, the age of the research objects and combined diseases led to some clinical heterogeneity among the articles.
Conclusions
RCT articles on the use of sacubitril/valsartan in AMI were retrieved using evidence-based medical research methods for this meta-analysis, and the RCTs were systematically evaluated and meta-analyzed. Sacubitril/Valsartan can inhibit ventricular remodeling after AMI, improve cardiac function, and reduce the incidence, readmission rate and mortality of adverse cardiovascular events after myocardial infarction. The shortcomings of this meta-analysis include the potential over evaluation of clinical therapeutic effects due to limitations related to the quality of the included articles, the number of articles, and biases. Thus, high-quality clinical studies with larger sample sizes need to be included in the future to confirm the conclusions drawn in the meta-analysis. In summary, the meta-analysis provides new therapeutic evidence for the adoption of sacubitril/valsartan in the clinical treatment of cardiac failure after AMI.
Acknowledgments
Funding: This study was supported by the Science and Health Joint Project of Chongqing (No. 2020GDRC016), and Social and People’s Livelihood Science and Technology Innovation Special Project of Banan, Chongqing (No. 2020030).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-210/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-210/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang XF, Ye M, Yan D, et al. Non-invasive ventilation improves hemorheology status in hypoxemic patients with acute myocardial infarction after PCI. J Geriatr Cardiol 2017;14:274-9. [PubMed]
- Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail 2016;18:1228-34. [Crossref] [PubMed]
- Docherty KF, Campbell RT, Brooksbank KJM, et al. Effect of Neprilysin Inhibition on Left Ventricular Remodeling in Patients With Asymptomatic Left Ventricular Systolic Dysfunction Late After Myocardial Infarction. Circulation 2021;144:199-209. [Crossref] [PubMed]
- Diao K, Wang D, Chen Z, et al. Rationale and design of a multi-center, prospective randomized controlled trial on the effects of sacubitril-valsartan versus enalapril on left ventricular remodeling in ST-elevation myocardial infarction: The PERI-STEMI study. Clin Cardiol 2021;44:1709-17. [Crossref] [PubMed]
- She J, Lou B, Liu H, et al. ARNI versus ACEI/ARB in Reducing Cardiovascular Outcomes after Myocardial Infarction. ESC Heart Fail 2021;8:4607-16. [Crossref] [PubMed]
- Liu Y, Liu H, Hao Z, et al. Efficacy and safety of different doses of tirofiban combined with ticagrelor on diabetic patients with AMI receiving in emergency percutaneous coronary intervention (PCI). Int J Clin Exp Med 2015;8:11360-9. [PubMed]
- Heyse A, Manhaeghe L, Mahieu E, et al. Sacubitril/valsartan in heart failure and end-stage renal insufficiency. ESC Heart Fail 2019;6:1331-3. [Crossref] [PubMed]
- Sauer AJ, Cole R, Jensen BC, et al. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev 2019;24:167-76. [Crossref] [PubMed]
- Sato K, Takahashi J, Hao K, et al. Temporal trends in the prevalence and outcomes of geriatric patients with acute myocardial infarction in Japan-A report from the Miyagi AMI Registry Study. J Cardiol 2020;75:465-72. [Crossref] [PubMed]
- Solomon SD, Vaduganathan M, L, Claggett B, et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2020;141:352-61. [Crossref] [PubMed]
- Myhre PL, Vaduganathan M, Claggett B, et al. B-Type Natriuretic Peptide During Treatment With Sacubitril/Valsartan: The PARADIGM-HF Trial. J Am Coll Cardiol 2019;73:1264-72. [Crossref] [PubMed]
- Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol 2017;5:333-40. [Crossref] [PubMed]
- Haynes R, Judge PK, Staplin N, et al. Effects of Sacubitril/Valsartan Versus Irbesartan in Patients With Chronic Kidney Disease. Circulation 2018;138:1505-14. [Crossref] [PubMed]
- McMurray JJV, Jackson AM, Lam CSP, et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 2020;141:338-51. [Crossref] [PubMed]
- Vaduganathan M, Claggett BL, Desai AS, et al. Prior Heart Failure Hospitalization, Clinical Outcomes, and Response to Sacubitril/Valsartan Compared With Valsartan in HFpEF. J Am Coll Cardiol 2020;75:245-54. [Crossref] [PubMed]
- Solomon SD, Jhund PS, Claggett BL, et al. Effect of Dapagliflozin in Patients With HFrEF Treated With Sacubitril/Valsartan: The DAPA-HF Trial. JACC Heart Fail 2020;8:811-8. [Crossref] [PubMed]
- He W, Cao M, Li Z. Effects of different doses of atorvastatin, rosuvastatin, and simvastatin on elderly patients with ST-elevation acute myocardial infarction (AMI) after percutaneous coronary intervention (PCI). Drug Dev Res 2020;81:551-6. [Crossref] [PubMed]
- Jering KS, Claggett B, Pfeffer MA, et al. Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure Events after Myocardial Infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail 2021;23:1040-8. [Crossref] [PubMed]
- Rezq A, Saad M, El Nozahi M. Comparison of the Efficacy and Safety of Sacubitril/Valsartan versus Ramipril in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol 2021;143:7-13. [Crossref] [PubMed]
- Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380:539-48. [Crossref] [PubMed]
- Wang H, Fu X. Effects of sacubitril/valsartan on ventricular remodeling in patents with left ventricular systolic dysfunction following acute anterior wall myocardial infarction. Coron Artery Dis 2021;32:418-26. [Crossref] [PubMed]
- Zhang Y, Wu Y, Zhang K, et al. Benefits of early administration of Sacubitril/Valsartan in patients with ST-elevation myocardial infarction after primary percutaneous coronary intervention. Coron Artery Dis 2021;32:427-31. [Crossref] [PubMed]
- Zaid Iskandar M, Lang CC. Sacubitril and valsartan fixed combination to reduce heart failure events in post-acute myocardial infarction patients. Drugs Today (Barc) 2017;53:545-51. [Crossref] [PubMed]
- Gatto L. Does sacubitril/valsartan work in acute myocardial infarction? The PARADISE-AMI study. Eur Heart J Suppl 2021;23:E87-90. [Crossref] [PubMed]
- Ishii M, Kaikita K, Sato K, et al. Cardioprotective Effects of LCZ696 (Sacubitril/Valsartan) After Experimental Acute Myocardial Infarction. JACC Basic Transl Sci 2017;2:655-68. [Crossref] [PubMed]
- Torrado J, Cain C, Mauro AG, et al. Sacubitril/Valsartan Averts Adverse Post-Infarction Ventricular Remodeling and Preserves Systolic Function in Rabbits. J Am Coll Cardiol 2018;72:2342-56. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)