New pulmonary rehabilitation exercise for pulmonary fibrosis to improve the pulmonary function and quality of life of patients with idiopathic pulmonary fibrosis: a randomized control trial
Introduction
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease with an unknown pathogenesis and chronic progression (1,2). The end stage IPF presents damaged alveolar structure, pulmonary fibrosis with honeycomb, gradually reduced lung volume, and progressively declined lung function (3). IPF progression adversely affects the quality of life and survival of patients (4). The average survival of patients with confirmed IPF is only 2 to 5 years (1,2,5). Except lung transplantation, effective therapies for IPF are still lacking (1,2). Thus, effective preventive and therapeutic method that can successfully improve lung function and quality of life and extend survival remains an unmet need.
The International IPF Guidelines emphasize that lung rehabilitation is one of the key therapeutic approaches for IPF (6). However, current lung rehabilitation focuses on physical exercise of whole body (7-13) and the perform is very complex. We developed the breathing exercises named “LHP’s Respiratory Rehabilitation for Pulmonary Fibrosis” (LHP’s RRPF, Registration No. 2015-V-00432628) specific for patients with pulmonary fibrosis. LHP’s RRPF designed based on the lung characteristics of patients with pulmonary fibrosis, such as reduced lung tissue elasticity, gradually decreased lung volume, and unable to tolerate intensive physical exercise because of hypoxia. In the current study, the safety and effectiveness of LHP’s RRPF on improving lung ventilation was first verified in healthy people, and then a randomized control trial (http://www.chictr.org.cn/enindex.aspx, Trial No. ChiCTR-OOC-15005818, study protocol was in online https://cdn.amegroups.cn/static/public/apm-21-71-1.pdf) to investigate the effect of LHP’s RRPF on lung function and quality of life of IPF patients was performed. We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/apm-21-71).
Methods
The breathing exercise movements, safety, and effectiveness of LHP’s RRPF
Breathing Exercise of LHP’s RRPF (Registration No.: 2015-V-00432628) includes 3 consecutive sets of movements (Figure S1):
- Deep breath of the whole lung;
- Deep breath of unilateral lower lung;
- Deep breath of the upper lung.
Detailed methods are in Supplementary file 1 (Appendix 1).
Lung ventilation efficiency test
Briefly, Vibration Response Imaging (VRI) (VRIxp, DEEPBREEZE) can measure lung function during breathing at resting state. The principle of operation of VRI is displayed in Figure S2. Patients’ maximal forced vital capacity (FVC) was used to estimate the lung volume of each lung section.
Safety and effectiveness in healthy individuals
Briefly, twenty healthy volunteers aged 40 to 50 (10 men and 10 women) completed the breathing exercises of LHP’s RRPF. VRI was performed before and after the breathing exercises to determine the effectiveness on lung ventilation.
Safety and effectiveness of LHP’s RRPF in IPF patients
Patient inclusion and exclusion criteria
Inclusion criteria: (I) patients were diagnosed with IPF (definite UIP) according to the 2013 and 2018 ATS/ERS/JRS/ALAT guidelines (14,15), and their condition was stable for at least one month; (II) 40 to 80 years of age, male or female; (III) were able to tolerate the breathing exercises; (IV) at stable state, arterial blood gas PO2 >60 mmHg and PCO2 <50 mmHg; (V) 6MWD ≥100 meters; (VI) had good compliance with the study protocol; (VII) were fully informed of study aims, methods, and potential adverse reactions, agreed to participate in the trial, and signed the informed consent form.
Exclusion criteria: (I) patients were unable to tolerate the breathing exercises; (II) had obvious pulmonary infection that required anti-infection treatment (patients had respiratory infection or systemic infection within 4 weeks of the enrollment); (III) had malignant tumors 5 years before the enrolment; (IV) participated in other clinical trials within 3 months of the enrolment; (V) had severe systemic diseases and organ dysfunction; (VI) were pregnant, nursing, planning for pregnancy, or unable to use effective contraceptive; (VII) had medical conditions that were considered unsuitable for the trial by the investigators.
The screening and single-blind randomization were completed at the Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study has been approved by the Institutional Review Board of Shanghai Pulmonary Hospital (approval No. K14-156). All patients enrolled in the study signed informed consent. Patients’ baseline data were collected and are presented in Table 1.

Full table
Study design and randomization
The sample size was calculated based on an estimated 5% improvement in FVC in lung function, and the estimated number of IPF cases was at least 44 for total, excluding the loss of follow-up rate of 10% patients. The study schedule is briefly displayed in Figure S3. Patients were randomly assigned to the LHP’s RRPF exercise (exercise group) or control group using sealed envelopes with randomized results by SAS analysis software (16), which were prepared and shuffled before the start of the study by an independent person unrelated to the study protocol. The envelope was opened by the allocator sequentially, only after the participant’s name was written on it. Both groups of the patients were scheduled to have clinic visits at the 6th and/or 12th month.
The patients assigned to the exercise group were trained to do the LHP’s RRPF exercise [Supplementary file 1 (Appendix 1) and Figure S1] and confirmed by investigator, then they attend a 12-month rehabilitation program. They repeated the exercise 3 times daily (4 to 6 minutes each time) and rested for 1 minute after each time. Patients continued their existing therapies during the trial at least for 1 year. The patients assigned to the control group were treated with their existing therapies, with basic medical care and an identical medical follow-up, similarly to those in exercise group. If patients developed acute exacerbation during the trial, the patients were withdrawn from the trial and treated immediately.
Effectiveness and safety assessment
Primary endpoints
Briefly, the study primary endpoints were changes in FVC and lung volume. Lung function was determined according to the 2005 ATS/ERS Guidelines (17). Changes in FVC after the breathing exercises were used to estimate the effectiveness of LHP’s RRPF. Chest X-ray was performed at the beginning and end of the trial. Lung volume was determined by the position of the left and right transverses relative to the position of the posterior rib on the X-ray image. Changes in lung volume after the breathing exercises were measured.
Secondary endpoints
Briefly, the study secondary endpoints included 6MWD (18), quality of life score (St. George’s Respiratory Questionnaire, SGRQ score) (19), forced expiratory volume in one second (FEV1) (17), and diffusing capacity of the lungs for carbon monoxide (DLCO).
Safety measurement
Electrocardiogram (EKG) was performed to assess the effect of the breathing exercise on cardiac function. Patients underwent EKG at the beginning of the trial and the 6th and 12th month clinic visits.
Statistical analysis
Paired t-test was used to compare the heart rate and blood oxygen saturation of healthy volunteers before the breathing exercise, right after the exercise, and one minute after the end of the exercise.
Independent sample t-test was used to analyze the baseline data of enrolled patients, including age, BMI, gender, disease duration, SGRQ score, 6MWD, FVC, FEV1, DLCO, and lung volume.
Unstructured covariance matrix was used in the linear mixed effect model (likelihood-ratio is the minimal), P<0.05 was considered significantly different. The statistical analysis software IBMSPSS24 was used.
Results
Effectiveness and safety of LHP’s RRPF in healthy volunteers
A total of 20 healthy volunteers participated in the breathing exercise. The lung volume of the healthy volunteers was monitored by VRI (QLD, MEF, EVP, Figure S4) during the exercise.
In addition, compared with the heart rate at resting state, the heart rate of every healthy volunteer was significantly increased immediately after completion of one- time breathing exercise, and the heart rate restored to the level at the resting state after the subject rested for one minute. Pulse oxygen saturation was not changed significantly by the exercise (Table S1).
In summary, those data support that LHP’s RRPF appears to be safe for healthy people and the three sets of movements of the breathing exercise can effectively expand the volume of different parts of the lung. These results indicate that the exercises might be suitable for IPF patients.
Effectiveness and safety of LHP’s RRPF in IPF patients
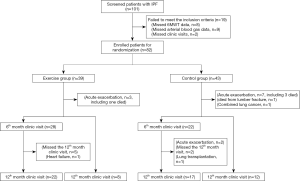
A total of 101 patients with IPF treated at the Department of Respiratory Medicine of Shanghai Pulmonary Hospital between January 2015 and May 2017 were screened. Of the 101 inpatients, 19 patients were excluded (2 patients can’t follow up, 8 patients’ 6MWT were <100 m, 9 patients’ arterial blood gas PO2 were <60 mmHg), 82 met the inclusion criteria and participated in the trial and were randomized to the breathing exercise group (39 cases) and non-exercise control group (43 cases). In the exercise group, 3 withdrew because of IPF exacerbation (one died), 28 finished the 6-month program and had 6th months clinic visit data, then 22 of 28 continually finished 12 months program and had the 12-month clinic visit data, 8 cases finished 12 months program but only had the 12-month clinic visit data. In the control group, 7 withdrew because of IPF exacerbation (three died); 1 withdrew because of death from lumbar fracture repair surgery; 1 withdrew because of lung cancer; 22 had the 6-month clinic visit data (17 of them had the 12-month clinic visit data); 12 only had the 12-month clinic visit data (Figure 1).
The two group patients showed similar baseline data (Table 1).
Results from the 6th month clinic visit
A total of 50 patients had the 6th month clinic visit data, including 28 patients in the exercise group and 22 patients in the control group. The average compliance rate of exercise group is 93.60%. The data are presented in Table 2. Although FVC at the 6th month of the trial reduced compared with the baseline values in both groups, the exercise group showed significantly less reduction in FVC than the control group (P=0.022, Figure S5A). SGRQ score has no difference between two groups. SGRQ score was reduced in the majority of patients in the exercise group (Figure S5B). The average changes in the SGRQ score (ΔSGRQ) was negative in the exercise group and positive in the control group, the absolute ΔSGRQ was significantly bigger in the exercise group (P=0.016). The 6MWD at the 6th month of the exercise group was similar to that at baseline, whereas the 6MWD of the control group was reduced (Figure S5C). The average Δ6MWD of the control group was a significant reduction from the baseline. The reduction in FEV1 at the 6th month of the exercise group was significantly less than that of the control group (P=0.017, Figure S5D). The average change in DLCO (ΔDLCO) was an increase in the exercise group, but was a reduction in the control group, and the absolute ΔDLCO was significantly greater in the control group than in the exercise group (P<0.001, Figure S5E). Thus, FVC, SGRQ, FEV1, and DLCO were significantly improved in the exercise group compared with the control group. The exercise group also showed improvement in 6MWD although with no statistical significance.

Full table
Results from the 12th month clinic visit
A total of 59 patients returned to the 12th month clinic visit (Table 3). The average compliance rate of exercise group is 94.71%. At the 12th month of the trial, Ten patients from the exercise group showed increased lung volume on chest X-ray (0.5–1, intercostal, Figure S6A). None of the patients in the exercise group had reduced lung volume at the 12th month of the trial. In contrast, 6 patients from the control group showed reduced lung volume (0.5–1 intercostal, Figure S6B). The exercise group showed significantly less reduction in FVC than the control group (P=0.001, Figure S7A,B). The average ΔSGRQ at the 12th month was negative in the exercise group and positive in the control group, and the absolute ΔSGRQ was significantly different in the two groups (P=0.003, Figure S7C,D). The average Δ6MWD was negative in both groups, and the reduction in 6MWD was significantly less in the exercise group than in the control group (P=0.041, Figure S7E,F). The average reduction in FEV1 was significantly less in the exercise group than in the control group (P=0.001, Figure S7G,H). The average reduction in DLCO was significantly less in the exercise group than in the control group (P=0.003, Figure S7I,J).

Full table
No new arrhythmias or other adverse events related to the LHP’s RRPF exercise were reported from the patient’s diary and follow-up electrocardiogram results. Of the 82 enrolled IPF patients, 3 from the exercise group and 9 from the control group developed acute exacerbation during the trial, but the difference was not significantly different (Table S2). The one-year mortality of the exercise group was lower than that of the control group although the difference was not statistically significant (Figure S8 and Table S3).
Discussion
The current IPF Diagnosis and Treatment Guidelines (2,6,16) state that no drugs can reverse the initiation and progression of fibrosis. The anti-fibrotic drugs that are currently commonly used in clinical practice, such as pirfenidone, nintedanib, and acetylcysteine. However, the effects of these drugs are not satisfactory (20-22). A previous study proved that lung rehabilitation was the most effective for patients with ILD, particularly for IPF (23). Lung rehabilitation for patients with IPF includes lung rehabilitation exercise (24), symptom management (25), lung rehabilitation education, and daily care (26). Lung rehabilitation exercises can improve cardiovascular function, effectively reduce IPF-associated cardiovascular morbidity and mortality (27), improve the physical activity and quality of life of patients with late stage IPF (28,29), significantly improve the scores of SGRQ-1 and International Physical Activity Questionnaire in 24 patients with IPF (30,31), and extend the duration of constant-load exercise in patients with moderate to severe IPF (32). Patients need to adhere to the exercise for a long time. When the exercises stop, patients’ exercise capacity reverses to the baseline level (33). Thus, it is necessary to develop breathing exercises that are safe, effective, easy to learn, cost effective, and suitable for patients with pulmonary fibrosis.
LHP’s RRPF was a set of breathing exercises and designed according to the characteristics of patients with lung fibrosis, such as reduced lung tissue elasticity, limited lung expansion, decreased lung volume, and substantially reduced vital capacity. Slowly repeating three of simple movements combined with deep breathing can practice the respiratory muscles time and again, maintain lung elasticity, slowdown lung volume reduction, delay lung function decline, reduce breathing difficulties, improve exercise endurance and quality of life.
In this trial, we first validated the safety and effectiveness of the breathing exercises on healthy volunteers by using VRI system to monitor the lung. Then we investigated the safety and effectiveness of LHP’s RRPF on patients with IPF. The control group was given medication and normal life, and was given regular guidance on disease rehabilitation (notes for disease attention, etc.). In view of the limitations of Chinese respiratory rehabilitation therapists, no patients received regular physical exercise or other rehabilitation exercises. In addition to the prescribed breathing exercises, the exercise group did not receive other breathing rehabilitation exercises. We found that at the 6th month of the trial, FVC reduction in the exercise group were significantly less than those in the control group, and SGRQ, FEV1, and DLCO improved significantly in the exercise group. The duration of the breathing exercise of our study is longer than that in the previous studies (10-13). At the 12th month of the trial, the reductions in FVC, FEV1, DLCO in the exercise group was significantly less than those in the control group, and these results are consistent with those from Swigris et al. (12). The breathing exercises can slow down the decline of lung functional and physical performance (6MWT) in IPF patients and improve the quality of life (SGRQ). After 12th month of breathing exercise, there was no breathing exercise-associated adverse event including EKG result indicating that breathing exercises is safe for IPF patients.
Our study has shown several advantages of LHP’s RRPF. (I) The design is scientific for pulmonary fibrosis patients, it is simple, and effective. Patients can easily learn the 3 sets of simple limb movements combined with deep breathing after being properly trained. During the exercises, the slow, gentle, and continuous movements can help patients to maintain lung elasticity, delay lung function decline, and improve quality of life. (II) The exercise is safe and has few side effects. IPF patients usually have limited physical activity capacity and some of them with hypoxemia, hence they may not be fit for excessive whole-body activity (7). LHP’s RRPF focus on exercising the respiratory muscles and minimally involve other muscles of whole body. Thus, the exercises did not cause excessive oxygen consumption. Duration of the exercises only need 4–6 minutes and can be tolerated well. Patients with hypoxia can perform the breathing exercise safely when they have oxygen inhalation. If patients have a cough, they can perform the exercises with antitussive drugs. (III) The exercises do not need any cost, because these breathing exercises do not require any specialized rehabilitation center and equipment. In addition, the quality control of patients in the process of exercise is the key to respiratory exercise, which needs to remind patients in the future clinical application, and strengthen the guidance and supervision of exercise in order to achieve better results.
Obviously, there was a certain rate of loss of follow-up in this study, and we also found that the consistency and standardization of breathing exercises were the difficulties in this study. In the process of follow-up, the changes of diseases and the difficulty of follow-up make us lose some patient data. In the exercise guidance of respiratory exercises, researchers are often required to supervise and guide patients’ exercise, so that patients can achieve the best exercise effect. In addition, for patients with moderate to severe IPF, especially those who cannot be separated from oxygen inhalation support, it is difficult for them to carry out breathing exercise. Therefore, this breathing exercise can only be carried out in patients with mild to moderate IPF.
Conclusions
In summary, LHP’s RRPF is safe, effective, easy to perform, no cost, and suitable for patients with IPF. The LHP’s RRPF may be an adjunct to pulmonary rehabilitation for IPF.
Acknowledgments
Funding: This study was funded by grants from the National Science Foundation of China (Grant No. 81730002, 81670055, 81670056, 91442103, 81500052, and 81570057), Ministry of Science and Technology of the People’s Republic of China (2016YFC1100200 and 2016YFC1100204), Shanghai Family Planning Commission Health Industry Clinical Research Project (20184Y0084), and Shanghai Hospital Development Center (16CR3054A).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-71
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-71
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-71). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of Shanghai Pulmonary Hospital (No. K14-156). All patients enrolled in the study signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frankel SK, Schwarz MI. Update in idiopathic pulmonary fibrosis. Curr Opin Pulm Med 2009;15:463-9. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis: An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. [Crossref] [PubMed]
- Schmidt SL, Tayob N, Han MK, et al. Predicting Pulmonary Fibrosis Disease Course From Past Trends in Pulmonary Function. Chest 2014;145:579-85. [Crossref] [PubMed]
- Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 2008;3:8. [Crossref] [PubMed]
- Raghu G, Rochwerg B, Zhang Y, et al. American Thoracic Society;European Respiratory society;Japanese Respiratory Society;Latin American Thoracic Association. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care 2015;192:e3-19. [Crossref]
- Spruit MA, Singh SJ, Garvey C, et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [Crossref] [PubMed]
- Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008;13:394-9. [Crossref] [PubMed]
- Bajwah S, Ross JR, Peacock JL, et al. Interventions to improve symptoms and quality of life of patients with fibrotic intersitial lung disease: a systematic review of the literature. Thorax 2013;68:867-79. [Crossref] [PubMed]
- Vainshelboim B, Oliveira J, Yehoshua L, et al. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration 2014;88:378-88. [Crossref] [PubMed]
- Vainshelboim B, Oliveira J, Fox BD, et al. Long-term effects of a 12-week exercise training program on clinical outcomes in idiopathic pulmonary fibrosis. Lung 2015;193:345-54. [Crossref] [PubMed]
- Swigris JJ, Fairclough DL, Morrison M, et al. Benefits of Pulmonary Rehabilitation in Idiopathic Pulmonary Fibrosis. Respir Care 2011;56:783-9. [Crossref] [PubMed]
- Rifaat N, Anwar E, Ali YM, et al. Value of pulmonary rehabilitation in with idiopathic pulmonary fibrosis. Egyptian Journal of Chest Diseases and Tuberculosis 2014;63:1013-7. [Crossref]
- Travis WD, Costabel U, Hansell DM, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-e68. [Crossref] [PubMed]
- Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care 2005;20:187-91; discussion 191-3. [Crossref] [PubMed]
- Miller A, Enright PL. PFT Interpretive Strategies: American Thoracic Society/European Respiratory Society 2005 Guideline Gaps. Respir Care 2012;57:127-33; discussion 133-5. [Crossref] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. Erratum in: Am J Respir Crit Care Med 2016;193:1185. [Crossref] [PubMed]
- Barr JT, Schumacher GE, Freeman S, et al. American Translation, Modification, and Validation of the St. George’s Respiratory Questionnaire. Clin Ther 2000;22:1121-45. [Crossref] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2093-101. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZCAPACITY Study Group, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Nakazawa A, Cox NS, Holland AE. Current best practice in rehabilitation in interstitial lung disease. Ther Adv Respir Dis 2017;11:115-28. [Crossref] [PubMed]
- Benton MJ, Graham HL. Maintaining quality of life in IPF patients: What role should pulmonary rehabilitation play? Respirology 2017;22:841-2. [Crossref] [PubMed]
- Garibaldi BT, Danoff SK. Symptom-based management of the idiopathic interstitial pneumonia. Respirology 2016;21:1357-65. [Crossref] [PubMed]
- Igai Y. End-of-life trajectory of coping and self-care of patients with idiopathic pulmonary fibrosis: a meta-synthesis using meta-ethnography. Jpn J Nurs Sci 2019;16:47-61. [Crossref] [PubMed]
- Vainshelboim B, Kramer MR, Fox BD, et al. Supervised exercise training improves exercise cardiovascular function in idiopathic pulmonary fibrosis. Eur J Phys Rehabil Med 2017;53:209-18. [Crossref] [PubMed]
- da Fontoura FF, Berton DC, Watte G, et al. Pulmonary rehabilitation in patients with advanced idiopathic pulmonary fibrosis referred for lung transplantation. J Cardiopulm Rehabil Prev 2018;38:131-4. [Crossref] [PubMed]
- Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res 2018;19:182. [Crossref] [PubMed]
- Arizono S, Taniguchi H, Sakamoto K, et al. Endurance time is the most responsive exercise measurement in idiopathic pulmonary fibrosis. Respir Care 2014;59:1108-15. [Crossref] [PubMed]
- Gaunaurd IA, Gómez-Marín OW, Ramos CF, et al. Physical activity and quality of life improvements of patients with idiopathic pulmonary fibrosis completing a pulmonary rehabilitation program. Respir Care 2014;59:1872-9. [Crossref] [PubMed]
- Jackson RM, Gómez-Marín OW, Ramos CF, et al. Exercise limitation in ipf patients: a randomized trial of pulmonary rehabilitation. Lung 2014;192:367-76. [Crossref] [PubMed]
- Vainshelboim B, Fox BD, Kramer MR, et al. Short-term improvement in physical activity and body composition after supervised exercise training program in idiopathic pulmonary fibrosis. Arch Phys Med Rehabil 2016;97:788-97. [Crossref] [PubMed]