
Intra-arterial chemoembolization with chemotherapy for unresectable locally advanced rectal cancer: a case report and literature review
Introduction
Rectal cancer is one of the most common malignant tumors globally. With the development of socioeconomics, especially changes in the diet structure, the incidence and mortality of rectal cancer are growing year by year (1). According to the latest global statistics, there were over 700,000 new cases and over 300,000 deaths in 2020 (1). Due to the lack of obvious symptoms in the preliminary stage, a great number of patients are already at clinical stage II and III when they are first diagnosed, which is defined as locally advanced rectal cancer (LARC) (2). For LARC, the standard treatment is neoadjuvant chemoradiotherapy and total mesorectal excision (TME), followed by postoperative adjuvant chemotherapy (3). Among these methods, TME surgery is the only approach which has demonstrated efficacy (4). However, after evaluation, only a small fraction of patients with LARC can receive surgery with R0 resection. For those who are not suitable or cannot tolerate surgery, the effect of palliative treatments is always unsatisfactory (5).
Intra-arterial chemotherapy (plus embolization) is a preoperative treatment applied to many malignant tumors, such as liver, pancreas, kidney, lung, cervix and breast etc. Indeed, better efficacies have been demonstrated in the above-mentioned tumors after the application of this method. But it has rarely been reported in primary rectal cancer. Since oxaliplatin is the most commonly used chemotherapeutic drug for transarterial chemoembolization (TACE) in liver cancer, we were planning to change the route of administration of oxaliplatin in rectal cancer. Recently, this method has been successful in treating several patients with unresectable LARC in our hospital. Here, we report a representative case who underwent this method. In addition, a concise review of the related literature is also presented. We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1881).
Case presentation
Patient information
Chief complaints
A 69-year-old female with a BMI of 21.32 kg/m2 was admitted to the Department of Gastrointestinal Surgery of our hospital on March 2, 2021, due to “vague hypogastralgia for about 1 year, and changes in bowel habit for about 3 months”.
History of present illness
About 1 year prior, the patient experienced pain at rest in the lower abdomen with no obvious causes. No specific treatment was taken during this period. About 3 months prior, the patient found that her stool became less in volume and unformed in shape with no obvious predisposing causes. The frequency of defecation was about 3–4 times a day. No treatment was taken during this period.
History of past illness
The patient had a history of chronic gastritis for several years and no treatment was taken.
Personal and family history
No specific personal and family history was provided.
Physical and endoscopic examinations
A rigid circumferential cauliflower neoplasm was detected in the rectum by digital rectal examination, and its inferior margin from the anal verge was about 6 cm. No blood remained on the fingertip after drawing the glove. The biopsy of the lesion performed via endoscopic examination demonstrated low differentiated rectal adenocarcinoma.
Laboratory examinations
The patient’s carcinoembryonic antigen (CEA) level was 18.27 ng/mL (reference value, 0–4.5 ng/mL) and cancer antigen 125 (CA-125) level was 338.45 U/mL (reference value, 0–35 U/mL).
Imaging examinations
Computed tomography (CT) and magnetic resonance imaging (MRI) were routinely performed. CT revealed that the rectal wall was irregularly thickened, and multiple lymph node metastases were present in the perirectal area, post-peritoneum, left pelvic wall, and left iliac region. MRI demonstrated that the inferior margin of the tumor from the anal verge was 8.1 cm, and the circumferential resection margin (CRM) was positive. In addition, the vessels outside the rectal wall had suspected invasion, and lymph node metastases were present in the mesorectum, lateral to the left internal obturator muscle, and the iliac region. Based on the above examinations, the tumor was evaluated clinically as cT4aN2M0, stage IIIB.
Treatments
After multidisciplinary treatment, neoadjuvant chemoradiotherapy was recommended to the patient for conversion therapy. Due to her financial situation and physical condition, the patient only chose chemotherapy for preoperative treatment. We recommended the mFOLFOX6 regimen to the patient according to the National Comprehensive Cancer Network (NCCN) guidelines. However, during the first chemotherapy treatment, which started on March 9, 2021, the patient had severe side effects of vomiting, despite tropisetron being routinely given. Multidisciplinary treatment was then carried out again to change the current regimen, and intra-arterial chemoembolization combined with CAPEOX chemotherapy was recommended and accepted by the patient. The procedures were conducted following the principles of the Declaration of Helsinki (as revised in 2013) and in accordance with the ethical standards of our hospital. The patient signed informed consent.
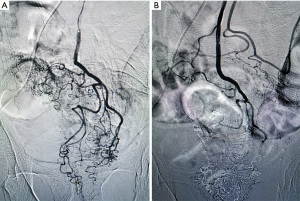
Two weeks later, intra-arterial chemoembolization was performed by our experienced surgeons. Under local anesthesia, the Seldinger technique was applied to insert the catheter from the right femoral artery into the aorta. After angiography using ultra-fluid lipiodol, the tumor vessels were displayed and superselected (Figure 1A). Then, a microcatheter was inserted into the inferior mesenteric artery via the catheter, and oxaliplatin (130 mg; based on the body surface area, 85 mg/m2) mixed with normal saline (50 mL) was infused into tumor arteries via the microcatheter, lasting for 10 minutes. After infusion, ultra-fluid lipiodol was injected for embolism. The entire operation ended after confirmation of embolism status under angiography (Figure 1B). Then, the patient underwent neoadjuvant chemotherapy with the CAPEOX regimen for 3 months, and no obvious side effects occurred during this period.
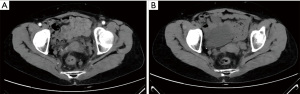
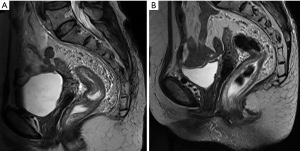
After 3 months of neoadjuvant chemotherapy with the CAPEOX regimen, CT and MRI were performed again to re-evaluate the local conditions of the tumor. Compared with the CT image before treatments (Figure 2A), we found in the current image that the surrounding lymph nodes of the tumor were reduced in number and volume (Figure 2B). And compared with the initial MRI image (Figure 3A), the tumor had also shrunk distinctly with a negative CRM in the current one (Figure 3B). We concluded that the tumor was resectable, and the patient met the conditions for the operation. Laparoscopic anterior resection with TME was planned for 8 weeks later, and the patient is now in the waiting period. After surgery, adjuvant chemotherapy with the CAPEOX regimen will be routinely given.
Discussion
Surgery is a key step in the standard treatment of LARC, and the ultimate purpose of preoperative treatments is to create good preconditions for radical surgery (6). Therefore, neoadjuvant chemoradiotherapy was firstly recommended for LARC in the NCCN guidelines in 2007. Compared with direct surgery, the advantages of neoadjuvant chemoradiotherapy as a preoperative treatment have been demonstrated. It promotes tumor downstaging and increases the R0 resection rate, which benefits postoperative survival (3).
However, many patients with LARC still fail to meet the operative conditions after neoadjuvant chemoradiotherapy. The reasons are closely related to some disadvantages of radiotherapy and chemotherapy. For radiotherapy, surgery can be difficult to perform after irradiation due to its scarring or fibrotic effects (7,8). Meanwhile, fluoropyrimidine-based chemotherapeutic drugs can act as radiation-sensitizers, thus aggravating the above impact on surgery (9). Consequently, some modified regimens have been proposed which lower the intensity of neoadjuvant chemoradiotherapy. Indeed, the FOWARC study (10) indicated that chemotherapy with or without radiation did not significantly improve the prognosis of patients with LARC. As for chemotherapy, its low local drug concentration and high systemic toxicity can lead to severe systemic failure without tumor remission (11). Additionally, since not all patients are sensitive to the selected drugs, disease progression or even distant metastasis may occur after such a long preparation (6 months recommended by the NCCN guidelines).
With the development of interventional medicine, intra-arterial chemotherapy has been applied in the treatment of many malignant tumors and has achieved promising results. For instance, as the routine approach for advanced primary hepatocellular carcinoma (HCC), transarterial chemoembolization (TACE) can significantly diminish the tumor volume and achieve satisfactory surgical conditions and prognosis (12). Based on the successful experience in HCC, we designed this interventional treatment for the present case.
In this case, intra-arterial chemoembolization as conversion therapy was carried out because of the late staging (cT4aN2M0) and poor operative field. The femoral artery is usually chosen for intubation to the primary tumor of LARC. Through regional catheterization, the tumor supply artery is superselected. High-concentration chemotherapeutic drugs immediately reach the artery and fully contact the tumors without any metabolism, thereby directly killing the tumor cells. This method increases the local drug concentration with lower systemic toxicity (13). Studies show that compared with systemic intravenous chemotherapy, regional intra-arterial chemotherapy can increase the drug concentration around and inside the tumors dozens of times (14,15). An increase in local drug concentration will correspondingly increase its effect, as tumors are very sensitive to its concentration (16). If it is doubled, the lethality on tumor cells can be correspondingly increased by more than 10 times (17). In contrast, the most significant feature of systemic intravenous chemotherapy is that drugs can stay in the body for a long time. However, it has been reported that the effect of chemotherapeutic drugs is most closely related to the concentration, but not to the staying time (18). Therefore, after intra-arterial chemotherapy with high-concentration drugs, the anti-tumor ability in local tumors is more prominent than intravenous administration. After infusion, the feeding arteries of tumors are usually embolized with gelatin sponges, lipiodol, or other commonly used embolic agents. Compared with infusion alone, adding embolization has the following advantages: (I) it can slow down the metabolism and excretion process of local drugs by slowing blood flow velocities, thereby delaying the rapid loss of drugs in a short period (19); (II) due to the “sandwich” sealing of the feeding arteries by embolic agents, drug concentration in the tumor tissues is greatly increased, while in the surrounding normal tissues it is much lower, thereby further improving drug effects and minimizing its toxicity to surrounding tissues (20); and (III) embolic agents can destroy the blood-supplying vessels and obtain the synergistic effect of cytotoxicity and ischemic necrosis (21).
Kimura et al. (22) reported a case of inoperable LARC in which the sacrum was invaded. Cisplatin and mitomycin were infused through the inferior mesenteric artery, followed by concurrent chemoradiation. The conditions for surgical resection were achieved, and R0 resection was successfully performed. Bini et al. (23) used chemoembolization with embolic drug-eluting beads containing irinotecan (DEBIRI) in 12 patients with LARC who were still unable to undergo surgery after standard treatments. The cancerous symptoms were alleviated to varying degrees. Among them, 4 cases had promising effects and the tumors were significantly reduced. Then, radical surgery was successfully performed. In fact, the volume of tumors is closely related to the prognosis of rectal cancer, and patients with larger tumors (77 cm3 as the critical point) have a higher risk of local recurrence and death (24). Huang et al. (25) reported 9 cases of locally bulky unresectable rectal cancer, the largest of which was 136 mm in size. After intra-arterial chemotherapy combined with concurrent chemoradiation, the volumes of the tumors in all cases significantly shrunk. Subsequently, R0 resection was performed successfully among these originally inoperable patients. Yang et al. (26) concluded that intra-arterial chemoembolization with oxaliplatin plus concurrent chemoradiotherapy as a neoadjuvant therapy provided a better pathological remission rate versus conventional treatments for LARC.
The above findings show that intra-arterial chemotherapy has a significant ability to kill tumor cells, reduce the volume of tumors, and remit local symptoms, thereby creating good operative conditions. Therefore, the above advantages of intra-arterial chemoembolization applied to patients with LARC may also deliver potential value in improving prognosis. In our prospective research, we aim to explore some long-term indicators such as local recurrence rate, distant metastasis rate, disease-free survival and overall survival. In addition, it is also our idea to take advantage of this method to reduce the frequency of preoperative treatments and partly replace neoadjuvant chemoradiotherapy, thereby improving the quality of life and prognosis of patients.
Conclusions
Preoperative intra-arterial chemoembolization is a novel approach for the treatment of patients with LARC. To the best of our knowledge, it has rarely been reported in western countries, and this is the first literature review summarizing the current situation of this method. This article may help in finding new approaches facing unresectable or inoperable LARC patients.
Acknowledgments
Funding: The study was supported by grants from the Natural Science Foundation of Yongchuan District, Chongqing (No. 2021yc-jckx20028) and the Project of Innovative Foundation for Postgraduates in Chongqing Medical University (No. YJSCX202012).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1881
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1881). All authors report that the study was supported by grants from the Natural Science Foundation of Yongchuan District, Chongqing (No. 2021yc-jckx20028) and the Project of Innovative Foundation for Postgraduates in Chongqing Medical University (No. YJSCX202012). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of our hospital and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yang J, Chen Q, Li J, et al. Short-Term Clinical and Oncological Outcome of Prolonging Operation Interval After Neoadjuvant Chemoradiotherapy for Locally Advanced Middle and Low Rectal Cancer. Cancer Manag Res 2020;12:2315-25. [Crossref] [PubMed]
- Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29-42. [Crossref] [PubMed]
- Body A, Prenen H, Lam M, et al. Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Recent Advances and Ongoing Challenges. Clin Colorectal Cancer 2021;20:29-41. [Crossref] [PubMed]
- Rothenberger DA. Palliative therapy of rectal cancer. Overview: epidemiology, indications, goals, extent, and nature of work-up. J Gastrointest Surg 2004;8:259-61. [Crossref] [PubMed]
- Abraha I, Aristei C, Palumbo I, et al. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst Rev 2018;10:CD002102 [Crossref] [PubMed]
- Sun Z, Adam MA, Kim J, et al. Optimal Timing to Surgery after Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. J Am Coll Surg 2016;222:367-74. [Crossref] [PubMed]
- Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34:3773-80. [Crossref] [PubMed]
- Keller DS, Berho M, Perez RO, et al. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol 2020;17:414-29. [Crossref] [PubMed]
- Deng Y, Chi P, Lan P, et al. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J Clin Oncol 2019;37:3223-33. [Crossref] [PubMed]
- Takiguchi N, Soda H, Tonooka T, et al. Utility of Preoperative Chemotherapy for Locally Advanced Colorectal Cancer. Gan To Kagaku Ryoho 2017;44:1296-8. [PubMed]
- Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293-313. [Crossref] [PubMed]
- Gao L, Cai S, Cai A, et al. The improved antitumor efficacy of continuous intratumoral chemotherapy with cisplatin-loaded implants for the treatment of sarcoma 180 tumor-bearing mice. Drug Deliv 2019;26:208-15. [Crossref] [PubMed]
- Maurer CA, Borner M, Büchler MW. Regional Chemotherapy of Gastro-Intestinal Cancer. Digestive Surgery 1997;14:9-22. [Crossref]
- Shchepotin IB, Chorny V, Hanfelt J, et al. Palliative superselective intra-arterial chemotherapy for advanced nonresectable gastric cancer. J Gastrointest Surg 1999;3:426-31. [Crossref] [PubMed]
- Fordy C, Glover C, Davies MM, et al. Hepatic arterial floxuridine as second-line treatment for systemic fluorouracil-resistant colorectal liver metastases. Br J Cancer 1998;78:1058-60. [Crossref] [PubMed]
- Sakai H, Ikeda S, Harada T, et al. Limitations of successive transradial approach in the same arm: the Japanese experience. Catheter Cardiovasc Interv 2001;54:204-8. [Crossref] [PubMed]
- Rowinsky EK, Noe DA, Trump DL, et al. Pharmacokinetic, bioavailability, and feasibility study of oral vinorelbine in patients with solid tumors. J Clin Oncol 1994;12:1754-63. [Crossref] [PubMed]
- Poon RT, Fan ST, Tsang FH, et al. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg 2002;235:466-86. [Crossref] [PubMed]
- Gao L, Li Q, Zhang J, et al. Local penetration of doxorubicin via intrahepatic implantation of PLGA based doxorubicin-loaded implants. Drug Deliv 2019;26:1049-57. [Crossref] [PubMed]
- Lewandowski RJ, Geschwind JF, Liapi E, et al. Transcatheter intraarterial therapies: rationale and overview. Radiology 2011;259:641-57. [Crossref] [PubMed]
- Kimura H, Shima Y, Kinoshita S, et al. Successful resection of locally advanced rectal carcinoma combined with preoperative chemoradiation. Hepatogastroenterology 2003;50:1393-5. [PubMed]
- Bini R, Comelli S, Leli R, et al. A novel approach to inoperable or recurrent rectal cancer by chemoembolization: A new arrow in our quiver? Oncotarget 2016;7:45275-82. [Crossref] [PubMed]
- Tayyab M, Razack A, Sharma A, et al. Correlation of rectal tumor volumes with oncological outcomes for low rectal cancers: does tumor size matter? Surg Today 2015;45:826-33. [Crossref] [PubMed]
- Huang W, Wu J, Liu G, et al. Chemoradiotherapy with Concurrent Regional Arterial Chemotherapy for Locally Bulky Unresectable Rectal Cancer: A Case Series. Oncol Res Treat 2019;42:678-83. [Crossref] [PubMed]
- Yang B, Shan J, Feng Y, et al. Transcatheter rectal arterial chemoembolization with oxaliplatin plus S-1 concurrent chemoradiotherapy can improve the pathological remission rate in locally advanced rectal cancer: a comparative study. Radiat Oncol 2020;15:94. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


