
Prolonged shedding of SARS-CoV-2 in an elderly liver transplant patient infected by COVID-19: a case report
Introduction
Coronavirus disease 2019 (COVID-19) pandemic gripped the globe. SARS-CoV-2 is highly infectious and is susceptible to all populations. Elderly, and patients with underlying comorbidities are prone to critical outcome (1). Immunosuppressed patients have greater risk for opportunistic infections. However, it is largely uncertain to determine if the incidence of COVID-19 is increasing in patients undergoing long-term immunosuppression. As far, reports upon COVID-19 infection in liver transplant (LT) patients are scare (2,3). Prolonged viral shedding is well described for other respiratory viruses in immunocompromised patients, and whether it is expected for SARS-CoV-2 remains unclear. Herein we present a case of prolonged shedding of SARS-CoV-2 in an elderly LT patient with COVID-19. No organs from executed prisoners were used in the manuscript. We present the following article in accordance with the CARE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-20-996).
Case presentation
On January 26, 2020 (day 1 of illness), a 61-year-old LT patient presented symptoms of fever and fatigue (timeline of the illness course is shown in Figure 1). He took ibuprofen and antibiotic amoxicillin at home, but the symptoms were unresolved. The patient went to the fever clinic and was admitted to the hospital on January 28 (day 3).

Upon admission, the laboratory findings revealed elevation of C-reactive protein (CRP) with normal lymphocyte count and liver function test. The etiology of common respiratory pathogens was negative. Chest computed tomography (CT) scan showed “small patchy shadows under the pleura and multifocal mixed ground-glass opacities (GGO)” on day 6 (Figure 2A). He was approached as a suspected case of COVID-19, then he was moved to an isolated unit. The patient has received immunosuppressive treatment for over 11 years and has a history of hypertension for 10 years. He is taking tacrolimus (TAC) monotherapy with tough concentration at 4–5 ng/mL. On day 11 of illness, repeated CT scan revealed extended scope of GGO. An oropharyngeal swab specimen was collected and tested positive by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay for the presence of SARS-Cov-2 RNA. The diagnosis of COVID-19 pneumonia was therefore confirmed. TAC monotherapy immunosuppression was discontinued. The patient received lopinavir (200 mg)/ritonavir (50 mg) (LPV/r) as two pills twice daily since illness day 10. Lianhua Qingwen Capsule (4 capsules three times per day, orally), a Chinese traditional medicine which presented inhibitory effect on SARS-CoV-2, was also provided. Moreover, antibiotic (piperacillin sulbactam, 5 g twice per day, intravenously) treatment was given to prevent secondary infection.
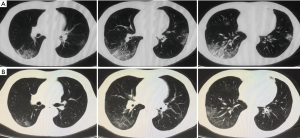
On day 13, the patient had complete symptoms relieve. The dynamics of CT scan showed marked absorption of pulmonary lesions on day 25 (Figure 2B). After temporary cessation for 14 days, TAC was restated at low dosage (0.5 mg twice per day, orally) and increased to full dosage (1 mg twice per day, orally) 1 week later. Laboratory tests for SARS-CoV-2 RNA from upper respiratory specimens had been performed repeatedly for surveillance. However, the shedding of coronavirus lasted a long time. Two consecutive negative SARS-CoV-2 RNA tests were displayed on day 35 and day 39 of illness. Exceptionally, the results turned positive again on day 41 and day 48 even after the resolution of symptoms. IgG antibody test for SARS-CoV-2 was positive with IgM negative on day 41. Then the patient was discharged on day 55 after confirmation with three consecutive negative results of SARS-CoV-2 RNA. He was advised to continue quarantine for at least 14 days, and received repeated examination during the period.
Discussion
As for immunosuppressed patients infected by COVID-19, there have been no large-scale data on a treatment guidance. In this study, TAC was discontinued since rapid development on pulmonary infiltration might probably result in acute respiratory distress syndrome (ARDS) in the elderly with a history of hypertension. Temporary cessation of immunosuppression may have a pivotal role in recovery of this patient, who had a speedy relieve of clinical symptoms. Indeed, immunosuppression may pose two opposing effects on COVID-19. It decreases the incidence of severe pneumonia by suppression of cytokine storm. On the other hand, it may prolong viral shedding time.
The understanding regarding the biological characteristics of SARS-CoV-2 in immunosuppressed patients is mostly unclear. In the present case, the laboratory test for SARS-CoV-2 RNA turned positive again on day 41 of illness after two consecutively negative results, while the symptoms had relieved up to 29 days. We surmised that the inconsistent situation of viral detection might be related to the causes as follow. First of all, laboratory test of the SARS-CoV-2 RNA depends on the viral load of the respiratory specimen, which is mostly sampled through oropharyngeal or nasopharyngeal swab. High viral load in the upper respiratory tract mainly appears in early stage of illness. In late phase or recovered stage, “false-negative” phenomenon may be presented on occasion due to lower viral load and the less expression of angiotensin-converting enzyme-2 (ACE-2) in the upper respiratory tract than in the lungs. It is indeed difficult to collect sputum and bronchoalveolar lavage samples from lower respiratory tract, which could increase the risk of aerosols transmission and is not recommended in this mild patient. Other attribution of false negative includes inexperienced operator or unstable quality of RT-PCR reagent kit. Secondly, it may attribute to delayed clearance of SARS-CoV-2 in the immunosuppressed patient. The median duration of virus shedding was 20 days, and the longest lasted 37 days, according to a study published by the Lancet (4). However, in the present case, the consecutive negative tests were shown on day 35 and on day 39 of illness, which may probably be false negative results. The final shedding duration was actually 52 days. As an elderly with pre-existing comorbidities, this immunocompromised patient was high risk for SARS-CoV-2 advocation. He presented prolonged shedding duration probably owing to long-term immunosuppression. To the best of our knowledge, high-dose steroids was associated with prolonged shedding of coronavirus (5), but it was not provided for treatment of this mild case. The impact of maintenance immunosuppression on the pathogenesis of SARS-CoV-2 infection is undefined as yet. Finally, the speculation about reinfection of SARS-CoV-2 in convalescence may be excluded by IgM negative test. Given the possibility of fluctuant results for SARS-CoV-2 RNA testing, we suggested that changes of sampling sites or multiple sampling from multi-sites could be more effective approaches to improve the positive rate of detection. Moreover, combination with IgM/IgG antibody test could be conductive to differential diagnosis.
In general, recovery of the patient with COVID-19 may contribute to temporary cessation of immunosuppression. Prolonged shedding of coronavirus should be a matter of concern and might attribute to long-term immunosuppression. Therefore, dynamic surveillance and prolonged quarantine are required for immunocompromised individuals. Indeed, our study is limited as a single case could not identify all the certainty and rationality. Further data should be collected to investigate if there is a universal prolonged shedding window of SARS-CoV-2 in immunosuppressed patients.
Patient perspective
The biological characteristics of SARS-CoV-2 in immunosuppressed patients are largely uncertain. From my perspective as a patient, the article by Wei et al. provides an interesting finding that immunosuppression may prolong viral shedding time. More cases should be collected to clarify the situation. I believe that repeated examinations and longer observation period may be needed for immunocompromised individuals after recovery from COVID-19.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-996
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-996). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This Study was approved by the institutional review board (IRB) at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB approval number:TJ-C20200120). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Qin J, Wang H, Qin X, et al. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology 2020;72:1491-3. [Crossref] [PubMed]
- Bhoori S, Rossi RE, Citterio D, et al. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol 2020;5:532-3. [Crossref] [PubMed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Auyeung TW, Lee JS, Lai WK, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect 2005;51:98-102. [Crossref] [PubMed]


