
Optimized stratification of risk factors in and beyond the CHA2DS2-VASc score to differentiate the real thromboembolic risk in mainland China: a systematic review and meta-analysis
Introduction
Atrial fibrillation (AF) is a dominant cause of morbidity and mortality worldwide, frequently leading to systemic thromboembolism and ischemic stroke (1,2). Strokes associated with AF are related to higher mortality and greater disability when compared with those from other causes (3). Moreover, the prevalence of AF among adults aged ≥40 years in mainland China was up to 2.31%, which was higher than the reported global scale (4). Thus, stroke prevention is central to the management of AF, regardless of rhythm control strategies (2).
The CHA2DS2-VASc score is currently considered as the cornerstone for the thromboembolic risk assessment and the anticoagulation therapy (5). Previous evidence suggested that the CHA2DS2-VASc score may be more excellent than other scoring systems in discriminating the risk of embolization (6,7). However, several limitations of the CHA2DS2-VASc score are observed gradually. Recent work has showed that the score may not be validated in an ethnically diverse population (8). Moreover, many of the less common stroke risk factors, beyond the CHA2DS2-VASc score, should be included in the score (9). Only two risk factors, gender and age, are currently assigned with different points according to each stratification in the CHA2DS2-VASc score. It would be simplistic to regard that all risk factors carry equal weight (9). The weight of each risk factor should be appropriately modified according to the different stratification. No previous study has investigated the stratification of risk factors in the score. Thus, the aim of this meta-analysis was to optimize the stratification of risk factors in and beyond the CHA2DS2-VASc score.
We present the following article/case in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-297).
Methods
Search strategy, inclusion and exclusion criteria, data extraction, outcomes of interest, quality assessment and statistical methods in the present meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISRM) guidelines.
Search strategy
PubMed, Embase, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), and Chinese Science and Technology Journal Database (VIP) were systematically searched from their inception to January 2020 for all studies regarding risk factors of nonvalvular atrial fibrillation (NVAF) with ischemic stroke in mainland China. The following terms were used in [Tittle/abstract]: “nonvalvular atrial fibrillation OR ischemic stroke AND risk factors AND mainland China”. To expand the search scope, we used the function of related articles and performed the manual search to get all reference lists of retrieved studies and conference abstracts. The most complete or recent literature was included if multiple literatures with the same population were found.
Inclusion and exclusion criteria
Independent reviewers (Wenjie Li and Lingling Xu, Tao Wang and Xiaojun Zeng) performed the literature screening according to selection criteria, titles, abstracts, and full-texts. Any discrepancies were resolved by discussion with the third reviewer (Bi-hui Luo).
All available prospective and retrospective trials that evaluated risk factors of NVAF with ischemic stroke in and beyond the CHA2DS2-VASc score, that were performed in mainland China and had at least three of the outcomes of interest mentioned in the next part of this article, were included. Studies that enrolled patients with valvular atrial fibrillation (including moderate to severe mitral stenosis or mechanical heart valve), animal experimental literatures, case reports and review articles were excluded.
Data extraction and outcomes of interest
By using the EndNote X9.3 system, two reviewers (Wenjie Li and Xiaojun Zeng) screened all identified documents and extracted information as follows: (I) title, author, study design, study site, year of publication, (II) sample size, mean age, follow-up duration, and (III) related items for outcomes of interest. If the outcome data were not available in the studies, we contacted the corresponding authors by email, with a reminder after one week.
The outcomes of interest were risk factors in the CHA2DS2-VASc score (age, female, hypertension, stroke/transient ischemic attack, diabetes mellitus, congestive heart failure, coronary heart disease and vascular disease), and risk factors beyond the score, including body mass index (BMI), smoking, drinking, use of anticoagulant drugs, total cholesterol (TC), triglyceride (TG), low/high density lipoprotein cholesterol (LDL-C/HDL-C), systolic blood pressure (SBP), diastolic blood pressure (DBP) and left ventricular ejection fractions (LVEF).
Quality assessment
The methodological quality of the included studies was accessed by using the Newcastle-Ottawa Scale (NOS), which included eight items (10). Each included study was scored out of a maximum of 8 scores. Studies with scores ≥7 were of high quality. Moderate-quality was judged if the scores were 4 to 6, and studies with scores <4 were of low quality.
Statistical analysis
All statistical analyses were performed by using RevMan 5.3 and Stata 15.0. Because of the large variety of patient characteristics, the odds ratios (ORs), weighted mean differences (WMDs) with standard deviations (SDs) and 95% confidence intervals (CIs) were obtained by using random-effects models even in case no heterogeneity was found. Single-arm analyses were conducted to evaluate the mean of SBP, DBP and LVEF, and the double-arm analyses were performed to evaluate the ORs and WMDs. Statistical heterogeneity was assessed by Cochrane Q test and I2 statistic. For Q test, P<0.1 (two-sided) was statistically significant. Heterogeneity was considered to be low if I2 was <50%; otherwise, it was high. Sensitivity analyses were conducted to assess the robustness of results by removing each included study individually. Potential publication bias was estimated by a funnel plot.
Results
Study selection
The database search yielded 3,629 Chinese articles and 1,968 English articles. Initial screening of these articles yielded 3,876 potentially qualified studies following the removal of 1,718 duplicates. After reviewing titles and abstracts, 40 articles were identified for full-text review, with 20 articles meeting full criteria (Figure 1).

Study characteristics and quality assessment
Table 1 outlines the characteristics of all the 20 observational studies. All publications were full-text literatures. These studies sourced from 14 regions in mainland China and included a total of 14,675 patients. Sample sizes ranged from 44 patients to 8,143 patients. All patients underwent antihypertensive therapy. The quality assessment demonstrated that no study was less than four scores. Six studies were of high-quality (11-16) and fourteen studies were of moderate-quality (17-30) by using the NOS.
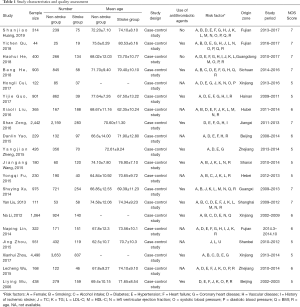
Full table
Results of meta-analysis
The assessment of all the outcomes is presented in Table S1. Most risk factors in the CHA2DS2-VASc score were significantly associated with ischemic stroke (hypertension: OR 2.06, 95% CI: 1.54–2.27, P<0.00001; age: OR 3.88, 95% CI: 2.20–5.56, P<0.00001; diabetes mellitus: OR 1.60, 95% CI: 1.30–1.98, P<0.0001; vascular disease: OR: 2.56, 95% CI: 1.19–5.48, P=0.02). However, female and congestive heart failure showed no statistical significance. Eight studies provided detailed data regarding the use of anticoagulant agents and showed a greater risk of stroke (OR 0.97, 95% CI: 0.86 to 1.10), but the difference did not reach statistical significance (P=0.65).
The mean SBP and DBP at baseline between the ischemic stroke group (SBP: 144.07 mmHg, 95% CI: 140.74–147.40; DBP: 84.41 mmHg, 95% CI: 82.39–86.43) and the non-stroke group (SBP: 132.99 mmHg, 95% CI: 131.86–134.12; DBP: 80.08 mmHg, 95% CI: 78.63–81.53) are shown in Figures S1-S4. Notably, the double-arm analysis demonstrated that compared with the non-stroke group, patients with the higher blood pressure (BP) level at baseline were significantly related to a higher incidence of ischemic stroke (SBP: WMD 10.98 mmHg, 95% CI: 7.80–14.17, P<0.00001; and DBP: WMD 4.46 mmHg, 95% CI: 2.57–6.35, P<0.00001) in the ischemic stroke group (Figures 2,3).


The single-arm analysis showed that the mean LVEF was 55.37% (95% CI: 52.40–58.33) in the stroke group and 58.41% (95% CI: 57.11–59.71) in the non-stroke group (Figures S5,S6). LVEF in the ischemic stroke group was 3.05% lower than that in the non-stroke group (95% CI: -5.96 to -0.14, P=0.02) (Figure 4) . Moreover, risk factors beyond the CHA2DS2-VASc score also demonstrated the statistical significance. The levels of TC (WMD 0.32, 95% CI: 0.04–0.61, P=0.02) and LDL-C (WMD 0.14, 95% CI: 0.02–0.26, P=0.02) in the ischemic stroke group were significantly higher than those in the non-stroke group (Figures S7,S8).

Sensitivity analysis and publication bias
Sensitivity analysis showed that none of the outcomes changed significantly and that the degree of heterogeneity decreased slightly. No obvious publication bias was found in the funnel plot of the studies that evaluated LVEF (Figures S9).
Discussion
The value of the CHA2DS2-VASc score for stroke risk prediction has been well documented (6,7). However, patients with NVAF might be of higher risks for left atrial thrombus (LAT) despite a low CHA2DS2-VASc score. The optimized weight of risk factors in and beyond the CHA2DS2-VASc score according to the stratification is relatively new (9,31). To the best of our knowledge, this is the first systematic review to optimize the stratification of risk factors in and beyond the CHA2DS2-VASc score. The major findings were as follows: (I) Patients with higher BP levels at baseline were significantly associated with a higher incidence of ischemic stroke. (II) HFpEF subgroup could be subdivided into heart failure with lower preserved ejection fraction (HFLpEF) and with higher preserved ejection fraction (HFHpEF), which represented different thromboembolic risks. (III) The addition of risk factors beyond the CHA2DS2-VASc score, TC and LDL-C, may improve the predictive performance of the score. 4) Female might not be an independent risk factor of thromboembolism for NVAF patients in mainland China. This finding was consistent with the latest data from China-AF study and the contemporary Japanese-AF guideline, which did not include female sex as an independent risk factor for anticoagulant treatment (32,33).
Hypertension is the most common risk factor for the development of AF worldwide (34). However, it remains unclear whether SBP and DBP levels are related to the risk of stroke in AF patients (35). In the present meta-analysis, the ischemic stroke group showed significantly higher BP levels and a greater risk of ischemic stroke than the non-stroke group. These results corroborated the finding of the previous trial (36) and suggested that AF patients with higher BP above the median values experienced a higher risk of stroke. Moreover, the Stroke Prevention in Atrial Fibrillation (SPAF) trial suggested that SBP ≥160 mmHg was independently related to the increased risk of stroke (37). Another analysis showed that the cutoff was elevated SBP ≥140 mmHg (38). The present study indicated that the mean SBP 144.07 mmHg (95% CI: 140.74–147.40) was at a significantly higher risk of ischemic stroke, with the elevated mean DBP 84.41 mmHg (95% CI: 82.39–86.43). This conceivably reflected a negative correlation between the elevated BP and the risk of ischemic stroke in NVAF patients. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline put forward a stricter definition of hypertension (SBP ≥130 mmHg or DBP ≥80 mmHg), inconsistent with the previous definition in the CHA2DS2-VASc score (39). The guideline also recommended treating SBP/DBP to <130/80 mmHg for AF patients (39). However, few studies have validated this recommendation and the predictive abilities of recalculated risk scores for ischemic stroke (40). In this meta-analysis, the mean SBP and DBP in the non-stroke group were 132.99 mmHg (95% CI: 131.86–134.12) and 80.08 mmHg (95% CI: 78.63–81.53), which may have implications guiding the target of antihypertensive therapy for ischemic stroke prevention and support anticoagulation recommendations. Of note, low BP reducing to <110/60 mmHg would lead to more adverse events (41). Based on the mean SBP and DBP, we produced some evidence on new BP cut-off values to predict ischemic stroke in patients with NVAF. Weigh of “H” in the CHA2DS2-VASc score could be optimized according to the stratification of SBP and DBP, not simply history of hypertension or uncontrolled BP.
In the present study, patients with congestive heart failure showed a greater risk of ischemic stroke, but the difference did not reach statistical significance. This may be attributed to the insufficient number of events. As recommended in the guideline, the diagnosis of heart failure mainly included symptoms, a clinical examination, NT-proBNP and transthoracic echocardiography to detect LVEF (42). Heart failure is currently defined as ‘with reduced ejection fraction (HFrEF)’and ‘with normal or preserved ejection fraction (HFnEF or HFpEF)’ (43). Of note, the definition of HFpEF is difficult, which can be defined by various classifications or inclusive criteria of clinical researches (ranging from ≥40% to ≥55%) (44,45). The unclear definition resulted in the heterogeneity of HFpEF patients in many researches. In addition, it is puzzling to note that HFpEF is a special group, featuring the highest CHA2DS2-VASc scores but the lowest risk of thromboembolic events (46). Such difficulties in classification influenced our study to some extent, but the present findings still added important insights into highlighting HFpEF definition for the modified CHA2DS2-VASc score. Based on our data, LVEF ranged from 50% to 60% in the majority of patients with HFpEF. Further analysis demonstrated that LVEF in the ischemic stroke group was lower than that in the non-stroke group. Given that, we hypothesized that HEpEF (LVEF: 50–60%) could be subdivided into lower preserved ejection fraction (HFLpEF) and higher preserved ejection fraction (HFHpEF). The risk of thromboembolism will be significantly great when LVEF reaches certain value between 50% and 60%. That is, HEpEF patients with HFLpEF and HFHpEF may identify quite distinct populations, which differ appreciably in terms of the thromboembolic risk (46). Although a body of researches are required to validate the best critical value, congestive heart failure in the CHA2DS2-VASc score is necessary to be modified according to the stratification of LVEF.
Interestingly, in the light of our data, indicators beyond the CHA2DS2-VASc score could also refine the scoring system. This was consistent with recent evidence which demonstrated that TC and LDL-C were independent predictors of NVAF with ischemic stroke (47,48). The underlying mechanism may be that cholesterol depletion resulted in impairment in cardiomyocyte contractility by deregulating adrenergic signaling, calcium handling and the myofibrillar architecture (49). More large-scale trials are required to investigate the best cut-off values of TC and LDL-C to predict ischemic stroke in patients with NVAF.
This study has potential limitations that must be emphasized. First, inclusion of observational studies may share an intrinsic risk for selection bias. However, it must be highlighted that there were no available randomized trials in this respect. Second, the studies included in the current meta-analysis comprised of a wider population in terms of regions, making the heterogeneity observed in the current meta-analysis. Third, the present systematic review was unable to offer specific cut-off values of the stratification due to a lack of data at the individual level. More researches are needed in the future.
Conclusion
This paper firstly proposed a framework for the optimized stratification of risk factors in and beyond the CHA2DS2-VASc score. The weight of risk factors in the CHA2DS2-VASc score, especially for hypertension and congestive heart failure, could be modified according to the stratification. In addition, TC and LDL-C may be independent predictors of NVAF with ischemic stroke. The addition of risk factors beyond the score could improve the predictive performance. These findings might be of great importance in differentiating patients who are potentially at high risks for ischemic stroke and LAT.
Acknowledgments
The authors would like to thank the department of cardiology of the First Affiliated Hospital of Guangzhou Medical University.
Funding: This work was supported by Natural Science Foundation of Guangdong Province of China (2018A030313060), Medical Science and Technology Research Foundation of Guangdong Province of China (A2018191, A2020178, A2020284) and Guangzhou Health Science and Technology Project (20201A011072), Guangzhou Medical University Project (B195001078).
Footnote
Reporting Checklist: The authors have completed the PRISRM reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-297
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-297). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837-47. [Crossref] [PubMed]
- Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet 2016;388:806-17. [Crossref] [PubMed]
- Lip GY. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J 2013;34:1041-9. [Crossref] [PubMed]
- Wang X, Fu Q, Song F, et al. Prevalence of atrial fibrillation in different socioeconomic regions of China and its association with stroke: Results from a national stroke screening survey. Int J Cardiol 2018;271:92-7. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Chao TF, Lin YJ, Tsao HM, et al. CHADS(2) and CHA(2)DS(2)-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol 2011;58:2380-5. [Crossref] [PubMed]
- Aspberg S, Chang Y, Atterman A, et al. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J 2016;37:3203-10. [Crossref] [PubMed]
- Golwala H, Jackson LR 2nd, Simon DN, et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. Am Heart J 2016;174:29-36. [Crossref] [PubMed]
- Szymanski FM, Lip GY, Filipiak KJ, et al. Stroke Risk Factors Beyond the CHA(2)DS(2)-VASc Score: Can We Improve Our Identification of "High Stroke Risk" Patients With Atrial Fibrillation? Am J Cardiol 2015;116:1781-8. [Crossref] [PubMed]
- Wells GA, Shea B, O’ Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed June 15, 2012.Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Huang SJ, Hong HS. Predictors of ischemic stroke in elderly non-valvular atrial fibrillation patients. Chin J Geriatr Heart Brain Vessel Dis 2019;21:261-5.
- Qu YC, Xu GY. Risk assessment on ischemic stroke in patients with non-valvular atrial fibrillation. J Mod Med Health 2018;34:1965-7.
- He B. Primary prevention and follow-up analysis of stroke in patients with non-valvular atrial fibrillation. Master's thesis, Xinan Medical University, China. 2018.
- Guo YJ, Wang HJ, Chen YX, et al. The risk factors analysis for ischemic stroke in Chinese patients with nonvalvular atrial fibrillation. Chin J Clin Healthc 2016;19:4-7.
- Xu SY. Retrospective analysis of influence factors for stroke in patients with non-valvular atrial fibrillation. Master's thesis, Guangxi Medical University, China. 2014.
- He HH. The predictive factor and its value in predicting ischemic stroke/TIA of patients with non-valvular atrial fibrillation. Master's thesis, Jinan University, China. 2018.
- Liu Y. The risk factors analysis for stroke in non-valvular atrial fibrillation patients with low-intermediate risk CHA2DS2-VASc score. Master's thesis, Fudan University, China. 2013.
- Cui HL, Ma DL. Research on clinical risk factors of patients with non-valvular atrial fibrillation and cerebral arterial thrombosis. China & Foreign Med Treat 2017;36:1-3,25.
- Liu XL, Guo ZL, Li P. Clinical characteristics and the analysis of the risk factors of nonvalvular atrial fibrillation with ischemic stroke. J Clin Med Pract 2016;20:162-3.
- Zeng S. The retrospective investigation of 2442 cases of hospitalized patients with nonvalvular atrial fibrillation. Master's thesis, Nanchang University, China. 2016.
- Zheng YJ, Lin HL, Wang WN, et al. Analysis of the risk factors of nonvalvular atrial fibrillation with ischemic stroke. Prev Treat Cardiovasc Cerebrovasc Dis 2015;15:468-70.
- Wang JG, Xi JT, Li JS, et al. The risk factor analysis of ischemic stroke prevalence in elder patients with non-valvular atrial fibrillation. Chin Cir J 2015;30:753-6.
- Yao DL, Mu HM. The clinical significance of the changes of serum uric acid content in patients with non-valvular atrial fibrillation and ischemic stroke. Chin J Clin Res 2015;28:1027-9.
- Fu YQ, Tian X, Geng W, et al. The study of CHADS2 score combined with red blood cell distribution width predicting the risk of ischemic stroke in patients with non-valvular atrial fibrillation. Chin J Med 2015;50:43-6.
- Li N, Ti M, Lu WH, et al. The risk factors analysis for stroke in patients with non-valvular atrial fibrillation. Chin Circ J 2012;27:189-91.
- Zhou J, Gao F, Bai HS, et al. Multifactor analysis of ischemic stroke in patients with non-valvular atrial fibrillation from northern Shaanxi region. Chin J Cardiovasc Rehabil Med 2013;22:553-6.
- Ruo Z, Zhou XH, Tang BP, et al. Analysis of risk factors in 4490 patients with nonvalvular atrial fibrillation. Chin J Cardiac Arrhyyth 2017;22:251-6.
- Lin NP. Relevant risk factors analysis of the stroke in the non-valvular atrial fibrillation. Master's thesis, Fujian medical university, China. 2015.
- Mu LY, Du FH, Xu XY. Clinical analysis of the risk factors of nonvalvular atrial fibrillation with ischemic stroke. J Capital Med Univ 2006;(3):375-8.
- Wu LC and Bao DG. An analysis of the factors leading to the illness of cerebral apoplexy in patients with nonvalvular atrial fibrillation. Health Res 2015;(3):287-9.
- Jagadish PS, Kabra R. Stroke risk in atrial fibrillation: beyond the CHA2DS2-VASc Score. Curr Cardiol Rep 2019;21:95. [Crossref] [PubMed]
- Lan DH, Jiang C, Du X, et al. Female Sex as a Risk Factor for Ischemic Stroke and Systemic Embolism in Chinese Patients With Atrial Fibrillation: A Report From the China-AF Study. J Am Heart Assoc 2018;7:e009391. [Crossref] [PubMed]
- Group JCSJW. Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2013). Circ J 2014;78:1997-2021. [Crossref] [PubMed]
- Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568-76. [Crossref] [PubMed]
- Ishii M, Ogawa H, Unoki T, et al. Relationship of Hypertension and Systolic Blood Pressure With the Risk of Stroke or Bleeding in Patients With Atrial Fibrillation: The Fushimi AF Registry. Am J Hypertens 2017;30:1073-82. [Crossref] [PubMed]
- Healey JS, Hart RG, Pogue J, et al. Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE-W). Stroke 2008;39:1482-6. [Crossref] [PubMed]
- Hart RG, Pearce LA, McBride R, et al. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 1999;30:1223-9. [Crossref] [PubMed]
- Rao MP, Halvorsen S, Wojdyla D, et al. Blood Pressure Control and Risk of Stroke or Systemic Embolism in Patients With Atrial Fibrillation: Results From the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. J Am Heart Assoc 2015;4:e002015. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018;138:e484-594. [PubMed]
- Kim D, Yang PS, Kim TH, et al. Ideal Blood Pressure in Patients With Atrial Fibrillation. J Am Coll Cardiol 2018;72:1233-45. [Crossref] [PubMed]
- Badheka AO, Patel NJ, Grover PM, et al. Optimal Blood Pressure in Patients With Atrial Fibrillation (from the AFFIRM Trial). The American Journal of Cardiology 2014;114:727-36. [Crossref] [PubMed]
- Bozkurt B, Aguilar D, Deswal A, et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016;134:e535-78. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Ho JE, Zern EK, Wooster L, et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation 2019;140:353-65. [Crossref] [PubMed]
- Kotecha D, Chudasama R, Lane DA, et al. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta-analysis of death and adverse outcomes. Int J Cardiol 2016;203:660-6. [Crossref] [PubMed]
- Siller-Matula JM, Pecen L, Patti G, et al. Heart failure subtypes and thromboembolic risk in patients with atrial fibrillation: The PREFER in AF - HF substudy. Int J Cardiol 2018;265:141-7. [Crossref] [PubMed]
- Roh E, Chung HS, Lee JS, et al. Total cholesterol variability and risk of atrial fibrillation: A nationwide population-based cohort study. PLoS One 2019;14:e0215687. [Crossref] [PubMed]
- Qi Z, Chen H, Wen Z, et al. Relation of Low-Density Lipoprotein Cholesterol to Ischemic Stroke in Patients With Nonvalvular Atrial Fibrillation. Am J Cardiol 2017;119:1224-8. [Crossref] [PubMed]
- Hissa B, Oakes PW, Pontes B, et al. Cholesterol depletion impairs contractile machinery in neonatal rat cardiomyocytes. Sci Rep 2017;7:43764. [Crossref] [PubMed]


