
Distal clavicle autograft—I would like to use my own osteochondral graft please
Introduction
Bone loss has emerged as a critical issue in the treatment of glenohumeral instability. Its presence has been reported in up to 72% of instability cases and is known to influence the outcome of surgical intervention. Many studies have correlated poor clinical outcomes, failure, morbidity and increased cost with arthroscopic soft tissue stabilization in the setting of significant bone loss.
Although glenoid bone loss in this setting is a poor predictor, the amount of bone deficiency that places an arthroscopic soft tissue repair at risk continues to evolve. Traditionally, bone loss greater than 20% has been shown to adversely affect biomechanical stability and clinical results. Utilizing Lo and Burkhart’s “inverted pear” concept of glenoid bone loss morphology, a defect as small as 6 to 8 mm can result in recurrent instability following soft tissue stabilization. Even in the absence of recurrence, sub-critical bone loss of 13.5% can be detrimental to clinical outcomes. More recently, greater interest has been paid towards the interaction of glenoid bone loss and the Hill Sachs lesion of the humerus. Larger Hill Sachs lesions may “engage” glenoid defects of all sizes in functional positions and have been shown to negatively affect arthroscopic results. The biomechanical and clinical effect of these two bone loss conditions have been combined into an “on-track, off-track” concept (1-4).
The recognition and treatment of bone loss are critical factors to ensure the successful management of anterior shoulder instability. Multiple bone grafting options exist to address this deficiency. Each approach has advantages and disadvantages unique to its application, which is covered in this review. The rationale, indications and technical notes for the senior author’s preferred technique of distal clavicle osteochondral autograft (DCA) are also discussed.
Surgical treatment options
There are numbers of options described to treat bone loss in shoulder instability. These include coracoid transfers such as the Latarjet and Bristow procedures, iliac crest graft bone grafting, and osteochondral allografts. Factors such as graft size, the presence or absence of articular cartilage, availability, immunocompatibility, and cost are all considerations in graft selection (Table 1). In addition to restoring the bone and cartilage loss seen in erosive glenoid bone loss, the ideal graft should also be readily available, free, and sourced without donor site morbidity.
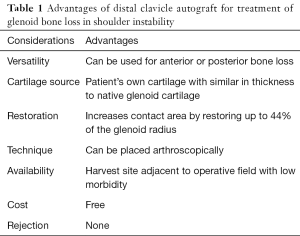
Full table
Coracoid bone autograft
In 1954, Latarjet first described his technique of coracoid bone transfer to the anterior glenoid for treatment of recurrent anterior dislocation of the shoulder. In 1958, Helfet proposed an alternative coracoid tip transfer and named it the Bristow technique. More than a half century later, the open coracoid transfer is still considered the gold standard technique for glenoid bone loss treatment. In addition to restoring glenoid articular bone, this technique offers further stabilization via capsuloligamentous reconstruction and inferior subscapularis myodesis from tightening of the conjoined tendon in the provocative position, which has become known as the “triple blocking effect”. Biomechanical studies demonstrate that this procedure is quite effective in restoring shoulder stability, and clinical outcomes studies have demonstrated low recurrence rates with excellent patient reported outcomes (5-8).
There are a number of drawbacks with this technique, however. First, coracoid transfer is a non-anatomic approach, which can make future revision surgery more difficult with an overall complication rate of up to 30%. Second, there is a limit to the amount of glenoid surface that can be restored. Paladini et al. demonstrated that this technique may be insufficient to restore defects exceeding 31% (9). Bueno et al. showed that, in comparison to the traditional Latarjet technique, the modified congruent arc technique may be used to reconstruct bone loss up to 54% of the glenoid surface (10). This modification, however, results in a greater graft displacement and a lower clinical failure load. Third, other authors have shown that up to 60% of the graft may undergo osteolysis (2,11). Fourth, the transferred graft lacks articular cartilage. This drawback has been cited as a potential reason for osteoarthritis development after Latarjet surgery, which can occur in up to 62% of cases (12). Finally, perhaps the most often cited concern regarding this technique is its associated complications. Unplanned reoperations have been reported at a higher rate than that of the traditional Bankart, and the overall complication rate has been reported as high as 25% (13,14). Delaney et al. published that neuromonitoring during Latarjet surgery resulted in a nerve alert in 77% of patients, and resulted in 21% of patients with a clinically detectable nerve deficit postoperatively (15).
Iliac crest bone autograft (ICBG)
The first reports of using a bone block to restore glenoid deficiency were made by Eden [1918] and Hybbinette [1932] (16,17). In 2006, Warner et al. reported autogenous tricortical iliac crest bone graft to be effective in treatment of recurrent instability in setting of glenoid bone loss (18).
The authors determined that this graft could restore defects up to 35 mm in length, which covers significantly more surface area than that of coracoid graft. They also reported excellent short-term results with a relatively low complication rate (4% of patients). The technique has the advantage of being readily available, essentially free, and is an autograft source of bone (18).
There are several potential drawbacks to this technique, however. First, the iliac crest is non-articular and thus cannot restore the osteoarticular loss seen in the glenoid. This may lead to secondary osteoarthritis which has been reported after this procedure (19,20). An additional drawback is the potential for donor side morbidity, from which persistent pain, greater than 1 year post-operatively, can occur in up to 100% of cases. Other complications such as local infection (14%) and anterior superior spine fracture (3%) incidences have been reported (18,21,22). Finally, like all free grafting techniques, the ICBG graft does not, in itself, address the soft tissue pathology that is frequently present in instability cases where it is utilized (23).
Distal tibia allograft
Distal tibial allograft has recently been introduced as an osteochondral source for glenoid bone loss treatment. Studies have shown that it can provide at least equivalent biomechanical properties to the iliac crest bone graft, and the technique has been shown to produce a better articular pressure profile than that of the Latarjet. Furthermore, some authors have demonstrated that glenoid arc articular conformity can be reproduced with this graft source (24,25). Promising clinical outcomes have been presented, but limited data has been published in the peer reviewed literature to this point (26).
This technique, however, has some limitations. While initial work has demonstrated that the distal tibia is well matched to glenoid articular anatomy, more recent studies have reported conflicting results. Decker et al. published that the chance of a random pairing of a distal tibial allograft matching the radius of curvature of a recipient glenoid is low (27). The degree of match precision necessary to achieve optimal results remains to be studied. The possibility of graft resorption secondary to immunologic response associated with this technique has not been investigated, but this concern has plagued allograft usage in other transplant settings (28-31).
The largest limitation to this method is its logistic application. The cost of a fresh osteochondral allograft can exceed tens of thousands of dollars, and there is often significant wait time which can exceed 6 months. Fresh allograft preparation requires a minimum of 14 days quarantine for infectious disease screening, and chondrocyte viability has been shown to significantly drop after 28 days post mortem. This limitation requires surgeons to perform transplantation in roughly a 2-week window, which can be a scheduling challenge for both patient and surgeon in many facilities (6).
DCA
Recently, Tokish et al. described a technique of employing the distal clavicle as a fresh, osteochondral autograft in the treatment of glenoid bone loss (32). The DCA is the first reported option that provides an autograft source of bone and cartilage to replace similar tissue loss on the glenoid. It has the advantage of being readily available and sourced with minimal cost. It also can be placed arthroscopically, as well as utilized in both anterior and posterior cases of bone loss. While donor site morbidity has not been reported with this specific technique, graft harvest is similar to the Mumford technique which is known to give excellent to good outcomes in up to 85% of treated patients. Dissatisfaction is typically correlated with over- or under-resection of the distal clavicle in the setting of arthritis. Researchers have previously suggested excising 5 to 10 mm of distal clavicle to optimize results, which is similar to our technique (33).
Recent work has shown that the DCA can, on average, reproduce up to 44% of the glenoid radius, which compares favorably to the 31% restoration achieved with the traditional coracoid transfer (5). Moreover, the distal clavicle graft is capped with articular cartilage which is within 1 mm of native glenoid cartilage thickness. It is a fresh, unprocessed tissue source that is immediately transplanted, so concerns about chondrocyte viability, immunorejection, or infection are minimized. The theoretical advantages of this technique are summarized in Table 1.
While promising anatomic results have been reported, there is a lack of clinical outcomes with this technique. Thus, concerns about recurrence rates, complications and long-term results, while initially promising in the senior author’s hands, have not been published in the peer-reviewed literature. However, supporting biomechanical data is available for this technique. Petersen et al. examined contact pressure differences between the clavicular grafting and congruent arc coracoid transfer techniques and determined a more favorable profile for the DCA (34).
It is the preference of the senior author to use the DCA in young patients who have glenoid bone loss as the primary reason for their instability, with defects from 15% to 30%, and relatively preserved soft tissue structures.
This technique also has several potential limitations. First, it does not augment or address anterior capsular structures that are often a part of complex instability cases. Thus, in cases of collagenopathies, such as Ehlers-Danlos syndrome, previous thermal capsulorrhaphy, or multiply operated patients, we prefer an alternative technique which may address these issues.
Author’s preferred surgical technique
Preoperative preparation
Patients with glenohumeral instability should undergo a standard history and physical examination, as well as preoperative advanced imaging such as CT or MRI. Glenoid bone loss is calculated in every patient, and this calculation aids in determining the operative approach to the patient according to the “on-track, off-track” concept. Relative measurement indications for bony augmentation in the setting of instability include bone loss greater than 15% of the glenoid diameter in the “off track” shoulder and significant retroversion in the context of posterior instability. Other factors such as age, athletic status, capsular laxity, and patient preferences are weighed when deciding between different treatment options.
Arthroscopic portal positioning
After the induction of general anesthesia, examination under anesthesia is performed to confirm the preoperative diagnosis. The patient is positioned in the lateral decubitus position on a beanbag with a padded axillary roll. The senior author prefers a padded arm sleeve (STAR sleeve; Arthrex, Naples, FL) with balanced suspension for limb positioning.
A standard posterior portal is established approximately 1-cm medial and 2-cm distal to the posterolateral acromial border. The arthroscope is introduced and additional portals are established using an outside-in technique under direct visualization with the use of a switching stick. The anterosuperior portal is established first, approximately 1 cm inferior to the clavicle and lateral to the coracoid. The mid-glenoid portal is created just superior to the superior border of the subscapularis. In cases of posterior augmentation, a 7-o’clock portal is positioned approximately 4 cm distal to the posterolateral corner of the acromion, bisecting the angle created by the posterior and lateral borders of the acromion. To allow efficient switching of the camera and instruments throughout the case, 8.25-mm cannulas are routinely used.
Diagnostic arthroscopy and biologic preparation
Following diagnostic arthroscopy while viewing from the posterior portal, the arthroscope is switched to the anterior-superior portal and a 3-mm graduated probe is placed to confirm our preoperative measurements for glenoid bone loss. Biologic preparation includes a wide release of the glenoid labrum to ensure its mobility for accurate reduction over and around the transplanted graft. Tissue is carefully mobilized with arthroscopic liberators and ablators. The glenoid is also biologically prepared with either an arthroscopic rasp or high-speed cylindrical burr, with the goal to create a healthy bed of bleeding cancellous bone, as well as to create a flat surface perpendicular to the glenoid margin to ensure a flush fit during graft placement.
Graft harvest
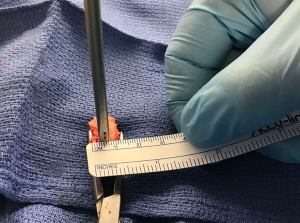
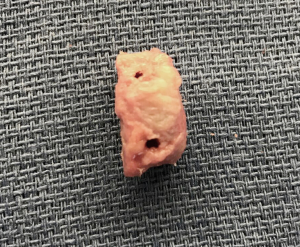
A single 3-cm horizontal incision is made over the subcutaneous border of the acromioclavicular joint, along the midline of the clavicular longitudinal axis. The skin and subcutaneous tissues are divided, and thick periosteal flaps are raised to expose the joint. A 1-cm wide saw blade is used to remove the distal approximate 1 cm of clavicle, and soft tissue is cleaned from around the bone. The graft is placed on the back table, and the harvest site periosteal flaps are closed with nonabsorbable No. 2 interrupted stitches. The remainder of the soft tissue is closed in two layers, and the wound is dressed at the completion of the case.
Graft preparation
The distal clavicle is a versatile graft, with a variable amount of version and an articular surface that is generally 19-mm long and 13-mm wide (Kwapisz, Tokish et al. unpublished data). The graft is evaluated based on its best fit and cut perpendicular to its articular surface to a width that matches the measurement of bone loss that was determined preoperatively and confirmed arthroscopically. In most cases, 7 to 8-mm of augmentation is normally sufficient to reconstruct up to 30% bone loss, and the graft is fashioned to anatomically fit and replace the loss. At this point, the method of fixation for the graft is chosen. While both screw or anchor fixation may be used, for anterior instability cases, anchor fixation is preferred. The angle for introduction required during drilling and screw advancement around or through the subscapularis can make screw fixation difficult. Prior to graft passage, two 1-mm drill holes are positioned 3 to 4-mm away from the articular surface at the superior and inferior borders of the graft, which will correspond to the planned offset position of the suture anchors or screws on the glenoid defect surface (Figures 1,2). For larger grafts, a third drill hole may be placed further from the articular surface to create an inverted triangle configuration.
Delivery and fixation of graft
Screw fixation
If the graft is to be fixed with screw fixation, it can be passed either freely into the joint or along a K-wire guide predrilled in the glenoid. The advantage of a free pass is that the graft may fit down a standard mid-glenoid cannula and, once inserted, can be flipped 90° and advanced through the rotator interval to match its resting position at the anterior-inferior glenoid. In this position, it can be held in place with a liberator introduced from the posterior portal. Likewise, the graft can be introduced through a posterior cannula and held in place with a liberator from the mid-glenoid portal. Wider exposure through the subscapularis is required to alternatively advance the graft down a predrilled K-wire and to properly position it on the glenoid. Once in place, a K-wire is placed through the pilot holes of the clavicle graft, and advanced into the native glenoid. This is usually not difficult for posterior grafts, but with anterior screw placement, the standard mid-glenoid portal may not be sufficient to achieve the appropriate angle. In such situations, an additional 5-o’clock portal is established through the subscapularis to ensure the correct trajectory. Care is taken to protect the axillary nerve, and if any doubt exists, it is dissected arthroscopically, visualized, and protected.
Once the graft is secured to the glenoid in the appropriate position with K-wires, a cannulated drill is advanced into the glenoid to allow lag fixation of the graft, with a cannulated, titanium 3.75-mm screw (Arthrex, Naples, FL). If the graft is too large to easily be delivered, the cannula can be removed, the portal expanded, and the graft delivered directly. If the proper trajectory cannot be achieved with wire provisional fixation, then one can consider using a suture anchor as an alternative or conversion to an open approach.
Suture anchor fixation
If suture anchor fixation is selected, the previously drilled holes in the graft are noted by their measurements from the articular surface and from each other. From these measurements, two 3.0-mm BioComposite SutureTaks (Arthrex, Naples, FL) are placed at the superior and inferior borders of the bone defect at the corresponding distances from the articular surface and each other, respectively (Figure 3). All limbs are delivered out of the working, mid-glenoid portal and shuttled through the corresponding holes in the graft (Figure 4). The graft is then manually introduced into the joint with a hemostat or small Kocher clamp through either a flexible Passport cannula (Arthrex, Naples, FL), half-slotted metal cannula employed as a sled, or freely through a cleared soft tissue portal. The graft is manipulated, positioned over the glenoid bone defect, and held securely in place with a liberator or probe from the posterior portal (Figure 5). One limb from each anchor is then tied to the other outside the mid-glenoid cannula for “double-pulley” delivery and fixation. The remaining free suture limbs from each anchor are then pulled to advance the pre-tied knot down the cannula and over the pre-positioned bone graft. Once the slack is pulled out of the anchor system, an arthroscopic square-knot is tied over the intervening bone bridge with three stacked half-hitches. Care is taken to ensure the graft remains in anatomic position while knots are tied (Figure 6).
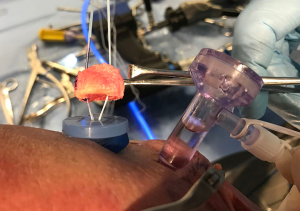

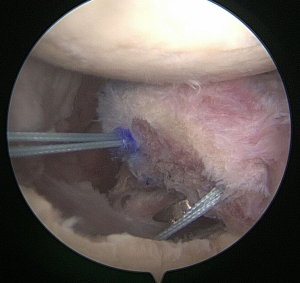
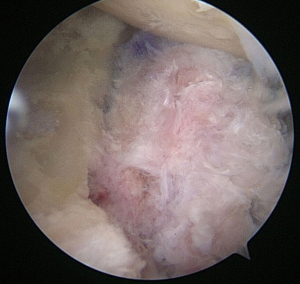
Incorporation of native labrum to graft
The tied suture limbs are passed through the native labrum in horizontal mattress fashion to bring it up to the neo-articular surface with the aid of retrograde suture lassos, and tied down with secondary stacked knots. If screw fixation has been used, supplemental suture anchors can be placed either through a graft of larger size or at the superior and inferior borders of the graft if there is concern for inadequate bony surface area. At conclusion, all arthroscopic instrumentation is removed, and the skin is closed and dressed sterilely.
Postoperative rehabilitation
The patient is placed in a neutral rotation sling for 6 weeks. Pendulums are allowed immediately, and passive motion is started at 3 weeks, with a goal to obtain full range of motion by 8 weeks. At 8 weeks’ follow-up, imaging is obtained, and if graft healing is noted, active motion is begun. Strengthening is added at 4 months postoperatively, and return to full activity is assessed at 6 months. Final radiographs are obtained at this point to ensure complete graft incorporation.
Conclusions
Glenoid bone loss in the setting of anterior shoulder instability can be addressed using a variety of techniques, each with unique advantages and limitations. In this review, we detail our preferred use of the DCA. This autograft provides a readily available and almost no-cost method for anatomical reconstruction of glenoid bone loss. The graft restores both the radius of the native glenoid and offers articular cartilage comparable in thickness to that of the native glenoid. It also compares favorably to the coracoid in terms of arc of restoration, providing a cortico-cancellous buttress for glenoid restoration. While this graft provides promising theoretical, anatomic, and biomechanical promises, longer term outcome studies are needed to validate its use in the clinical setting.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Albert Lin and Jason J. Shin) for the series “Trends in Anterior Shoulder Instability” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.10.12). The series “Trends in Anterior Shoulder Instability” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000;16:677-94. [Crossref] [PubMed]
- Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from "engaging/non-engaging" lesion to "on-track/off-track" lesion. Arthroscopy 2014;30:90-8. [Crossref] [PubMed]
- Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649-56. [Crossref] [PubMed]
- Kurokawa D, Yamamoto N, Nagamoto H, et al. The prevalence of a large Hill-Sachs lesion that needs to be treated. J Shoulder Elbow Surg 2013;22:1285-9. [Crossref] [PubMed]
- Giles JW, Boons HW, Elkinson I, et al. Does the dynamic sling effect of the Latarjet procedure improve shoulder stability? A biomechanical evaluation. J Shoulder Elbow Surg 2013;22:821-7. [Crossref] [PubMed]
- Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am 2013;95:1390-7. [Crossref] [PubMed]
- Neyton L, Young A, Dawidziak B, et al. Surgical treatment of anterior instability in rugby union players: clinical and radiographic results of the Latarjet-Patte procedure with minimum 5-year follow-up. J Shoulder Elbow Surg 2012;21:1721-7. [Crossref] [PubMed]
- Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy 2007;23:1033-41. [Crossref] [PubMed]
- Paladini P, Singla R, Merolla G, et al. Latarjet procedure: is the coracoid enough to restore the glenoid surface? Int Orthop 2016;40:1675-81. [Crossref] [PubMed]
- Bueno RS, Ikemoto RY, Nascimento LG, et al. Correlation of coracoid thickness and glenoid width: an anatomic morphometric analysis. Am J Sports Med 2012;40:1664-7. [Crossref] [PubMed]
- Di Giacomo G, Costantini A, de Gasperis N, et al. Coracoid graft osteolysis after the Latarjet procedure for anteroinferior shoulder instability: a computed tomography scan study of twenty-six patients. J Shoulder Elbow Surg 2011;20:989-95. [Crossref] [PubMed]
- Allain J, Goutallier D, Glorion C. Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am 1998;80:841-52. [Crossref] [PubMed]
- Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg 2013;22:286-92. [Crossref] [PubMed]
- Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am 2012;94:495-501. [Crossref] [PubMed]
- Delaney RA, Freehill MT, Janfaza DR, et al. 2014 Neer Award Paper: neuromonitoring the Latarjet procedure. J Shoulder Elbow Surg 2014;23:1473-80. [Crossref] [PubMed]
- Eden R. Zur Operation der habituellen Schulterluxation unter Mitteilung eines neuen Verfahrens bei Abriss am inneren Pfannenrande. Langenbecks Arch Surg 1918;144:269-80.
- Hybbinette S. De la transplantation d’un fragment osseux pour remédier aux luxations récidivantes de l’épaule: Constatations et résultats opératoires. Acta Chir Scand 1932;71:411-45.
- Warner JJ, Gill TJ, O'hollerhan JD, et al. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med 2006;34:205-12. [Crossref] [PubMed]
- Bouju Y, Gadea F, Stanovici J, et al. Shoulder stabilization by modified Latarjet-Patte procedure: results at a minimum 10 years' follow-up, and role in the prevention of osteoarthritis. Orthop Traumatol Surg Res 2014;100:S213-8. [Crossref] [PubMed]
- Hovelius L, Sandstrom B, Saebo M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg 2006;15:279-89. [Crossref] [PubMed]
- Almaiman M, Al-Bargi HH, Manson P. Complication of anterior iliac bone graft harvesting in 372 adult patients from may 2006 to may 2011 and a literature review. Craniomaxillofac Trauma Reconstr 2013;6:257-66. [Crossref] [PubMed]
- Calori GM, Colombo M, Mazza EL, et al. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury 2014;45:S116-20. [Crossref] [PubMed]
- Kleiner MT, Payne WB, McGarry MH, et al. Biomechanical Comparison of the Latarjet Procedure with and without Capsular Repair. Clin Orthop Surg 2016;8:84-91. [Crossref] [PubMed]
- Frank RM, Shin J, Saccomanno MF, et al. Comparison of glenohumeral contact pressures and contact areas after posterior glenoid reconstruction with an iliac crest bone graft or distal tibial osteochondral allograft. Am J Sports Med 2014;42:2574-82. [Crossref] [PubMed]
- Provencher MT, Ghodadra N, LeClere L, et al. Anatomic osteochondral glenoid reconstruction for recurrent glenohumeral instability with glenoid deficiency using a distal tibia allograft. Arthroscopy 2009;25:446-52. [Crossref] [PubMed]
- Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy 2017;33:891-7. [Crossref] [PubMed]
- Decker MM, Strohmeyer GC, Wood JP, et al. Distal tibia allograft for glenohumeral instability: does radius of curvature match? J Shoulder Elbow Surg 2016;25:1542-8. [Crossref] [PubMed]
- Aponte-Tinao LA, Ayerza MA, Muscolo DL, et al. What Are the Risk Factors and Management Options for Infection After Reconstruction With Massive Bone Allografts? Clin Orthop Relat Res 2016;474:669-73. [Crossref] [PubMed]
- Lozano-Calderon SA, Swaim SO, Federico A, et al. Predictors of soft-tissue complications and deep infection in allograft reconstruction of the proximal tibia. J Surg Oncol 2016;113:811-7. [Crossref] [PubMed]
- Chapovsky F, Kelly JD 4th. Osteochondral allograft transplantation for treatment of glenohumeral instability. Arthroscopy 2005;21:1007. [Crossref] [PubMed]
- Stevenson S, Li XQ, Martin B. The fate of cancellous and cortical bone after transplantation of fresh and frozen tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am 1991;73:1143-56. [Crossref] [PubMed]
- Tokish JM, Fitzpatrick K, Cook JB, et al. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech 2014;3:e475-81. [Crossref] [PubMed]
- Strauss EJ, Barker JU, McGill K, et al. The evaluation and management of failed distal clavicle excision. Sports Med Arthrosc 2010;18:213-9. [Crossref] [PubMed]
- Petersen SA, Bernard JA, Langdale ER, et al. Autologous distal clavicle versus autologous coracoid bone grafts for restoration of anterior-inferior glenoid bone loss: a biomechanical comparison. J Shoulder Elbow Surg 2016;25:960-6. [Crossref] [PubMed]
Cite this article as: Choate WS, Kwapisz A, Tokish JM. Distal clavicle autograft—I would like to use my own osteochondral graft please. Ann Joint 2017;2:77.


