
Feasibility of lung cancer screening in developing countries: challenges, opportunities and way forward
Introduction
Lung cancer is the most commonly diagnosed cancer type in the world with 2.094 million (11.6%) new cases of all diagnosed cancer cases with high mortality accounting for 1.8 million deaths (18.4%) in 2018. A total of 1,368,524 and 725,352 new lung cancer cases were reported in men and women respectively. The trend of lung cancer has changed over the decades but it is still a leading cause of death among men (1,2).
There is a rise in cases of lung cancer among women which is a major concern. Hungary is on the top of the list of lung cancer incidence among women, followed by other regions such as Northern America, Northern & Western Europe and Chile (3). Smoking, a major risk factor of lung cancer, accounts for about 85% of all lung cancers in current or former smokers (4), but this is changing now. Lung cancer cases among non-smoker females is becoming an important concern in developing countries (5,6).
Since most lung cancers are diagnosed at a late stage (7), lung cancer survival remains poor, not exceeding 15% at 5 years (8). Routine lung cancer screening is currently not recommended. Several studies have reported detection of lung cancer at an early stage with improved survival by making use of Low dose Computer Tomography in lung cancer screening. International Early Lung Cancer Action Program (I-ELCAP) results have shown a 10-year survival of 88% in patients with stage I lung cancer, which were identified during screening (9). A reduction of 20% was seen in deaths due to lung cancer in National Lung Screening Trial (NLST) with low dose computed tomography in comparison with chest radiograph. Three annual CT scans were conducted in NLST (10). Such outcomes demonstrate that lung cancer screening using CT can detect disease at a curable stage. It has been demonstrated that 90 percent of lung cancer cases can be attributed to smoking in developed countries, with the risk increasing with quantity and duration of smoking (11).
However, the epidemiology of lung cancer may be different in developing countries (12). While the prevalence of smoking (13), air pollution and environmental hazards (14) are considered to be significantly higher in developing countries, up to 30–40% of Asian lung cancer patients had never been smokers, in contrast to only 10% of patients in the United States (15).
Developing countries have a very high incidence of pulmonary tuberculosis and other chest infections (16-18). Therefore, misdiagnosis is a major concern (19-22). Lung cancer screening is largely restricted to developed countries in spite of high prevalence of lung cancer cases even in developing countries. It is showing a rising trend because of tobacco use, environmental pollution along with various other factors. There are frameworks for cancer screening in many of the developing countries but lung cancer screening is not included in spite of high incidence of lung cancer. This may be attributed to lack of infrastructure, no willingness for screening among high risk population, fear of disease, over-diagnosis, continuum of care for treatment and psychological impact (23-25). Tobacco control and smoking cessation is the major focus as primary prevention, but it seems difficult to enforce the existing rules and policies. This review will give an overview of lung cancer screening methods, challenges in implementation, existing guidelines and recommendation, newer point of care technology in addition to specific problems of developing countries where patients with pulmonary tuberculosis and chest infections are very large in number.
Lung cancer: trend and mortality in developing countries
Incidence of lung cancer has significantly increased in last three decades and has a worrisome increase in developing countries. In 1990, incidence of lung cancer was high in the developed countries but now around every three cases out of five are being diagnosed in developing countries (1,26). As per GLOBOCAN 2018, 58.5% of all lung cancer cases were from Asia, followed by Europe and North America with 22.4% and 12.4% cases, respectively. This may be attributed to population of Asia region with prevalence of habit of smoking cigarette, Bidi, Hukka, indoor and outdoor air pollution (27,28). Mortality due to lung cancer is high among men and most deaths occurs in developing countries of Eastern Europe, Western Asia, Northern Africa, Eastern and South-Eastern Asia. Incidence rate among men is high in Micronesia followed by Eastern Europe and Eastern Asia. Lung cancer incidence in Chinese females is similar to developed countries (3).
Burden of lung cancer is very high in Asia with an estimated incidence of over 1.2 million cases and approximately 1.07 million deaths. Lung cancer incidence is on top of the list among males, but it ranks third in women after breast and cervical cancers in Asia (1). Highest incidence rate of lung cancer was reported in South Korea China, Turkey, Singapore, Philippines (2,29). An estimated 774,323 new cases were reported in China with a mortality of 690,567 people (1). China accounts for almost half of the total cases of lung cancer worldwide. Furthermore, the incidence of lung cancer in China is increasing, with more number of young lung cancer patients (30).
In India, lung cancer ranks fourth (5.9%) in overall cancer incidence and second among males, while it ranks third in mortality (8.82%) due to cancer after breast cancer and head & neck cancer (1). According to Population Based Cancer Registries Report 2016, incidence of lung cancer is higher in North-Eastern Region of India where incidence varies from 3.22–28.25 cases, followed by southern region, eastern region and northern regions. Incidence is low in western and central region as compare to other regions in India. Despite variation in incidence geographically, lung cancer is the leading cancer in many cancer registries in India (31,32), also the cases of non-smoking lung cancer in India is on the rise. Increase of lung cancer incidence in Indian women is another worrying trend (5,6).
In African continent, lung cancer is the fourth most common cancer among men with approximately 39,300 new lung cancer cases and an estimated mortality of approximately 37,700 people per year. Northern Africa has the highest incidence of Lung cancer cases in Africa. There is a wide variation in incidence and mortality across the African continent (1,33). In South Africa, a decrease in mortality among men while an increase was seen among women during the period of 1995–2006 (34).
In Latin America and Caribbean Region, 89,772 new lung cancer cases were estimated with 51,757 cases in men and 38,015 cases in women in the year 2018. It is the third common cancer in this region causing an estimated 81,384 deaths. In addition, the 5-year prevalence is low at 13.11% (1).
Lung cancer is common among men in countries of medium human development index (HDI), low income and low middle income countries, but the recent trends show an increase in lung cancer cases among women too. This may be attributed to second-hand smoke exposure, environmental pollution; household pollution (1,2,35-37). Apart from high incidence, mortality is very high too, accounting for around two third deaths in developing countries out of the total lung cancer deaths of which around 60.7% of deaths are in Asia alone (1).
Lung cancer screening
Screening is an effective method to detect cancer at an early stage. While there are regular screening recommendations for breast and cervical cancer, it is not the same in case of lung cancer (38). Most countries or organisations have not framed any guidelines for lung cancer screening due to cost effectiveness and morbidity issues related to low-dose computed tomography (LDCT) (39). Various methods have been tried like chest radiography, sputum cytology, however, low dose computer tomography has been shown to be an effective screening modality for lung cancer.
Screening with chest radiography and sputum cytology
Lung cancer screening started in sixties when Brett published mass lung cancer screening research with approximately 55,000 men who were divided in two groups. Chest radiography was done biannually in test arm whereas chest radiograph was taken at starting and end of the study in control arm. There was no difference in mortality between test and control arms after three years of study period (40).
Three cooperative studies were conducted on lung cancer screening by National Cancer Institute, USA with Johns Hopkins Institute, Mayo Clinic and Memorial Sloan-Kettering Cancer Center to see the use of sputum cytology and chest radiograph in lung cancer screening. A total of 30,000 men participated in this study at 3 centers. In Mayo Clinic, half of the participants (screen group), were subjected to a dual-screen i.e., chest radiograph and sputum examination every 4 monthly for 6 years and another half were control. Participants were subjected to dual screen and chest radiography annually in each arm in Johns Hopkins and Memorial Sloan-Kettering. Participants who were screen negative at initial screening were followed up for 5 years or more. In Mayo Clinic study, chest radiograph detected lung cancer six times more when compared to sputum cytology, with a 5-year survival of 40% and 15% in screened and control groups respectively. This study could not show significant difference in mortality due to lung cancer like John Hopkins and Memorial Sloan-Kettering study where no benefit was seen in reducing lung cancer mortality by annual chest radiogram and sputum examination (41-45).
In PLCO randomised trial, 154,901 men and women aged 54–74 years were included from 1993–2001 at 10 screening centers. Chest radiograph was performed at the start of study followed by annual examination for three years. However, the results showed no reduction in lung cancer mortality (46).
Another study in Czechoslovakia compared the benefits of semi-annual lung cancer screening to 3-year interval screening with chest radiograph. Men aged 40–60 years who consumed 150,000 cigarettes or more in lifetime, current smoker were included in this study. Chest radiography was done biannually in one arm for 3 years, while sputum examination was done at starting and end of the study in another arm. There was no significant difference in survival in both arms (47). Chest radiograph and sputum examination is not recommended for lung cancer screening as none of the studies have shown survival benefit. Additionally, adding sputum examination to chest radiograph for lung cancer screening is not useful (42-45,47).
LDCT
There are many studies where lung cancer screening was tried with low dose computed tomography. National Lung Screening Test (NLST) was largest landmark study on lung cancer screening which changed the screening guidelines for lung cancer by showing 20% reduction in mortality due to lung cancer using LDCT in comparison to chest radiograph. This USA study included 53,000 participants into two groups in which annual LDCT was done in one group while single chest radiograph was done in another group. Positive screening rate was higher in LDCT in comparison to chest radiograph with high rate of false positive cases (10).
NELSON trial (Dutch-Belgian Lung Cancer Screening Trial), the largest European trial with 15,822 participants aged 50–75 years with a history of smoking 15 or more cigarettes per day for 25 or more years or 10 or more cigarettes per day for 30 or more years, current smoker or former smoker who had quit smoking less than 10 year ago were included in this trial. In contrast to NLST, control group in NELSON study received no chest radiograph, while screening group was subjected to LDCT screening. Total 4 rounds of screening were done at 0, 1, 3 and 5.5 years (48). The protective value of LDCT screening was more pronounced in women than in men. Overall, mortality in high risk men and women decreased by 26% and 61% respectively by low dose CT scan over a period of 10 years (49).
Canada conducted its LDCT trial in 2000, which included current or former smokers aged 50–74 years with a history of 30 pack years or more. A total of 21 lung cancer cases were detected. The use of a sputum biomarker in addition to LDCT has increased the detection of lung cancer. Low dose CT has the benefit of detecting more lung cancer cases and has increased detection rate form 3% to 5% (50).
A pilot randomized control trial of comparing LDCT with chest radiograph was conducted in France in the year 2002. The screening was done at the start of the study, followed by annually screening for two year. This study included current or former smokers aged 50–75 years consuming 15 or more cigarettes per day for 20 or more years. If LDCT detects nodules of size 5–10 mm, LDCT was repeated after three months to observe the changes. PET scan with or without histological examination was planned for nodules greater than 10 mm. A total of 765 participants were selected for randomization. It led to detection of 152 and 21 cases of non-calcified nodules on LDCT and chest radiograph, respectively, while 8 lung cancer cases were detected on LDCT out of 9 cases (51).
Lung cancer screening pilot trial in UK using LDCT was conducted in 2010. A total of 4,000 participants were divided into two arms. Participants aged 50–75 years, with a 5-year lung cancer risk of 5% or more based on Liverpool Lung project risk model were selected. One CT scan was performed at baseline and read by two experts who were supposed to record nodule size 3 mm or more in maximum diameter. Participants with nodule size of 10 mm or more on LDCT were referred to a local multidisciplinary team. Out of 1,994 participants who underwent LDCT, 42 were diagnosed with lung cancer. Only one LDCT screening in 5-years among population with lung cancer risk was found to be cost effective. Population based approach using validated risk assessment has the possibility to detect lung cancer at an early stage. It should however be noted that the prevalence of baseline lung cancer was high in UK trial in comparison to NLST (52).
A randomized control trial using LDCT was started in Denmark in 2004, in which a total of 4,104 participants aged 50–70 years, current smokers or minimum smoking history of 20 pack-years were randomised to two groups, the first with screening with five annual low dose CT scans and the second being no screening group. This trial reported no statistically significant effects on lung cancer mortality, but more number of early stage cancers (stages I, II and stage IIIa) were found in the screening group in comparison to the control group (53).
Japanese study using low dose CT scan was conducted in 1993 on 1,369 members of Anti-Lung Cancer Association (ALCA). Most of the members were men aged more than 50 years with smoking history of minimum 20 packs per year. LDCT was done once, twice, thrice and four times on 258, 318, 609 and 184 members respectively. This study concluded that low dose CT scan was better than chest radiography in the screening of lung cancer in high risk population (54).
There were trials using LDCT in Italy, South Korea, Germany & Taiwan and results are discussed in Table 1 (51,55-62). There are many on-going trials on LDCT for lung cancer screening in developing countries listed in Table 2.
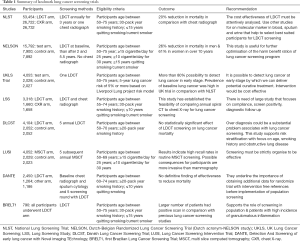
Full table
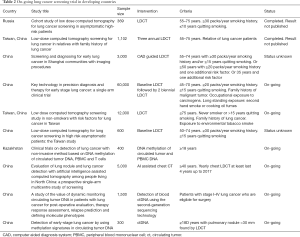
Full table
Screening in developing countries: existing programs & intervention
Though the burden of lung cancer is higher in developing countries, effective lung cancer screening program is not in place because of logistic issues. Several studies have been conducted worldwide for effective lung cancer screening methods (10,48-62). Most of these studies were conducted in developed countries and LDCT was used as standard lung cancer screening method. Several organisations have recommended use of LDCT in lung cancer screening for high risk population after mortality benefit of 20% shown in NLST study (10,63,64). But, cost of LDCT, risk of radiation, false positive finding and over-diagnosis make it difficult to implement at mass level (63). Considering the high incidence of lung cancer, China and Brazil conducted lung cancer screening trials using LDCT scans in 2014 and 2013–2014, respectively.
First Brazilian Lung Cancer Screening Trial (BRELT1) was conducted from January 2013 to July 2014. Around 4,030 participants showed interest but only 790 participants were taken in the program. Inclusion criteria for participants were same as NLST of USA with history of 30 packs-year smoking, current smoker or those who had quit smoking within last 15 years and aged between 55–74 years. The exclusion of large number of participants was because of lack of proper exposure of smoking (65). A LDCT was performed and pulmonary nodes more than 4 mm were taken as positive, similar to NLST trial. Positive findings were higher in BRELT1 (39.5%) in comparison to NLST (26%), but number of lung cancer cases found were almost similar in both studies. Though positive findings were higher in BRELT1, most of the large nodules had a very low suspicion of lung cancer in Brazil (65). Lung cancer screening is not recommended routinely in Brazil due to issues with applicability and effectiveness of LDCT. Incidence of granulomatous disease is high in Brazil and many pulmonary nodules may be attributed to tuberculosis and other chest infections (66).
In China, lung cancer screening trial was conducted by Cancer Institute of the Chinese Academy of Medical Sciences (CICAMS) in 2014 on 2,700 participants in three cities (67).
Screening challenges, opportunities & future research
Now, it is a well-established fact that screening for lung cancer with LDCT reduces mortality, a sign of relief in statement of three cooperative studies of Johns Hopkins Institute, Mayo Clinic and Memorial Sloan-Kettering Cancer Center in USA, which showed no benefit from lung cancer screening with chest radiograph and sputum cytology in decreasing the mortality (45). But still there are no framework or guidelines for lung cancer screening in developing countries, with LDCT or other screening method, due to various constraints in implementing such program.
Symptoms of lung cancer and Tuberculosis are overlapping as fever, cough, expectoration, anorexia and weight loss are common to both but history of smoking, hoarseness of voice and SVC obstruction points towards a diagnosis of lung cancer. It is not quite uncommon that both tuberculosis and lung cancer are misdiagnosed due to radiological similarity (68), additionally patients with tuberculosis are at risk to develop lung cancer (69,70). There are a large number of pulmonary tuberculosis cases in developing countries and India reported an incidence of 204 per 100,000 cases in 2017 (71). It’s always challenging and difficult to develop an effective lung cancer screening program in India in view of a large number of cases of pulmonary tuberculosis and other chest infections.
High false positive rate due to benign intrapulmonary lymph nodes or non-calcified granulomas, over-diagnosis and radiation exposure which leads to radiation induced cancer in long term are the harmful consequences of LDCT (10), and it remains the most critical issue with use of LDCT in lung cancer screening (72). Computer aided diagnosis (CAD) technique has high sensitivity in detecting lung cancer nodules with comparatively low specificity (73) and this system should be utilized in clinics for lung cancer screening. There is a decrease in false positive results in lung cancer screening with every millimetre increase in threshold nodule size (74). NLST, PLCO and other trial data showed that annual lung cancer screening reduced lung cancer mortality by 11–21% (range: 4.3–39.1% across various models) while biennial screening reduced only 6.5–9.6% lung cancer deaths. Triennial screening has limited scope in reducing lung cancer mortality. False positive results were increased with more frequent LDCT screening (75).
Over-diagnosis has been a problem in lung cancer screening, and ranges from 8.7–13.5% of screen detected lung cancer (75), but rate of over-diagnosis is low in LDCT in comparison to chest radiograph (10). LDCT has high sensitivity and specificity in detecting lung cancer among high risk smokers. Thus, selection of participants is the most important factor for cost effective and efficient screening program. Molecular Biomarker will be a value addition for lung cancer screening and may reduce the cost of lung cancer screening (50) but more scientific research and validation is required to make it a point of care technology for high risk individuals.
Population based approach using validated risk assessment has the possibility to detect lung cancer at an early stage using LDCT as shown in UK trial. This approach can be utilized to reduce the cost of lung cancer screening (52). Selection of eligible population for lung cancer screening is always a challenge in developing countries in view of perception and stigma related to tobacco and smoking in the society. These are the barriers for high risk population to participate in lung cancer screening (76).
Conventional screening chest radiography in Lung cancer screening has shown no positive results in various studies (77,78), but use of digital chest radiography, with computer aided diagnostic technique and highly quantum-efficient detectors tools, to improve visualization of pulmonary structures (79-85) may, therefore, be a more better and sensitive screening tool in detecting lung cancer than conventional chest radiography. Lung cancer screening with chest radiography is difficult and missing lung cancer lesions by radiologists is not uncommon (86). Special training to read the chest radiograph for lung cancer screening can be beneficial (87). This is not only cost effective but also using digital radiograph may reduce mortality. Digital chest radiograph is easily accessible and cost effective method with low radiation exposure to participants. LDCT has a much higher sensitivity in lung cancer screening for the detection of small nodules, but lack of financial resources add difficulties in implementing lung cancer screening using LDCT.
Lung cancer mortality reduction among males in some developing countries can be attributed to anti-tobacco policies, which had shown trend of reduction in tobacco use and smoking, a major risk factor of lung cancer, with ban on smoking in public places & public transport and increased taxes on cigarettes and other tobacco products (34,88), but still lack of comprehensive policies on control of smoking and lack of guidelines on promotional advertisement and activates of tobacco products, can increase the lung cancer incidence in developing world, due to increased smoking and tobacco consumption, as reported in Global Youth Tobacco Survey from 1999–2008 (89-92).
Tobacco control and smoking cessation remain the most important long term intervention to decrease morbidity and mortality from lung cancer in developing countries (93). There is a national framework for screening common cancers i.e., breast, cervical and oral cancers in India. Lung cancer is the most common cancer in males and its incidence is increasing further, but lung cancer screening is still not included in national framework in view of issues with availability of low dose CT scan and associated cost, also implicated is the high prevalence of pulmonary tuberculosis leading to over-diagnosis (38).
There is a need for aggressive research in lung cancer screening modalities for developing countries. There are many on-going trials for lung cancer screening in developing countries listed in Table 2 (94). These trials have inclusion criteria of more than 30 years of smoking history, current or past smokers who had quit smoking within 10 or 15 years with variable age scales, with or without additional risk factors as history of occupational exposures of carcinogens, second hand smoke and household combustion of coal. These trials are focusing not only on LDCT based intervention, but also on newer modalities like molecular biomarkers, which may help in reducing the screening costs with high sensitivity and specificity, along with advantage of being non-invasive and easy to implement. End results of these trials will certainly help developing countries to adopt a lung cancer screening method, if there is a proven survival benefit.
Newer modalities in lung cancer screening
Positron emission tomography (PET)
PET is a propitious technique for lung cancer screening. Two studies evaluated the patients with non-calcified lung lesions more than 7 mm in diameter on annual low-dose CT followed by PET with fluorodeoxyglucose (FDG) (95,96).
In a study by Bastarrika et al., FDG-PET correctly identified 19 of 25 indeterminate nodules. Sensitivity, specificity, positive predictive value and negative predictive value of using FDG PET for diagnosis of lung cancer were 69%, 91%, 90% and 71% respectively. Repeat CT was done after 3 months of negative FDG-PET, the negative predictive value was 100% (97). These results are promising but the obstacles to incorporation of FDG PET are cost and accessibility of FDG-PET. FDG-PET as a lung cancer screening tool needs to be validated in larger cohort studies.
Molecular biomarkers
Many research findings have demonstrated that, prior to lung cancer diagnosis, hypermethylation of gene promoters remains present in exfoliated cells within sputum. Promoter of hypermethylation of multiple genes, especially p16 ink4a promoter and p53 mutation are shown to appear in chronic smokers or high risk group before the clinical evidence of lung cancer (98-101). Telomerase activity in sputum may be helpful in differentiating benign from malignant tumours (102).
Autofluorescence bronchoscopy (AFB)
This technique identifies the areas of epithelial thickness and hyper vascularity as abnormal fluorescence and helps to improve sensitivity to diagnose pre-invasive lesions, squamous dysplasia, carcinoma in situ (CIS) and early lung carcinoma when used simultaneously with conventional bronchoscopy. AFB has shown usefulness to adjunct conventional bronchoscopy for detecting intraepithelial neoplasms and CIS as shown in single center studies, 3 multicenter and 2 randomized clinical trials (103-107). However the specificity of AFB is too low to diagnose the pre-invasive lesions. New autofluorescence imaging (AFI) has been introduced to increase the specificity that can distinguish the pre-invasive lesion and benign tumour by colour (108).
Electronic nose
Many volatile organic compounds (VOCs), especially alkanes and benzene derivatives have been identified in breath of lung cancer patients. According to a research study, for stage 1 lung cancer, which had 22 breath VOCs, showed 100% sensitivity and 81.3% specificity. Patients with and without lung cancer can be distinguished by using this technique (109-111). Electronic nose has been successfully used in detection and analysis of VOCs in the food industry. Various studies reported the use of this tool for VOC pattern analysis to detect lung cancer with fairly high diagnostic accuracies (111-118). However, no large scale implementation studies using electronic nose have been reported.
Genomic and proteomic analysis of bronchoscopic samples
The advances that have been made in understanding the molecular mechanisms of NSCLC progression may open the door for improvement of current therapeutics and identification of novel targets (119). A proteomics is an approach that clarifies the molecular steps involved in lung cancer development. This technique differentiates the pre-invasive bronchial lesion from invasive bronchial lesion by the specific patterns of protein expression of the airway epithelium, however, large scale studies are required to prove its validity (120).
Breath print analysis
This technique captures a signature of the whole exhaled breath that consists of a large number of non-selective sensors combined in sensor arrays (121-123). These multiple sensor arrays produce a multidimensional output, after that it is analysed with pattern recognition techniques specific to multivariate data analysis.
Lung cancer screening guideline and recommendations
Various organizations have given recommendations for lung cancer screening and a few countries have guidelines related to it, but there is no guideline for lung cancer screening in developing countries. International Association for the Study of Lung Cancer (IASLC) issued statement for LDCT based Lung cancer screening after promising results of NELSON study, with a focus of identifying the high risk population and developing radiological guidelines of lung cancer screening program which can be implemented in developing countries (124,125). Japan Radiological Society and Japanese College of Radiology have issued guideline for persons over 50 year with smoking history of 30 pack years (126,127).
National Cancer Center, Saudi Arabia has issued guideline for Lung Cancer Screening with annual LDCT for person aged 55–77 years with more than 30 pack year smoking history or those who had quit smoking less than 15 years ago (128). Canada also has recommended LDCT based lung cancer screening for persons aged 55–74 years with more than 30 pack years smoking history, current smoker or had quit smoking for less than 15 years. There is no recommendation of chest radiograph for lung cancer screening (129). European Union has also issued a position statement for lung cancer screening in Europe (130).
In USA, many organizations have issued guidelines for lung cancer screening, highlighting the high risk groups, and also age and frequency of screening. After survival benefit in NLST, American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Society of Clinical Oncology, American Lung Association, National Comprehensive Cancer Network, and US Preventive Service Task Force have recommended lung cancer screening for persons with smoking history of 30 pack year or more. But American Academy of Family Practice found insufficient evidence to recommend for or against screening (131-138). Details of guidelines and recommendations are listed in Table 3.
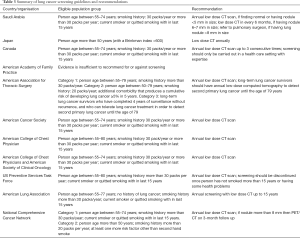
Full table
Conclusions
Incidence of lung cancer has significantly increased over the last three decades and has a worrisome increase in developing countries. LDCT has become the gold standard for lung cancer screening after survival benefits seen in NLST and NELSON studies. An effective lung cancer screening program is still a challenge in developing countries despite a high incidence of lung cancer. LDCT could be a good choice for screening, however, high cost of LDCT, large population size to be screened and low success rates of LDCT make it difficult to implement such a program. Also inadequate infrastructure, lack of human resources, low skilled manpower and lack of financial resources add further difficulties in adopting such a program. An Ideal Screening Method for developing countries should be easily and widely available, easy to perform and must be cost effective. High incidence of tuberculosis in developing countries further compounds the problem by adding to false positive cases during screening. There is a need to develop point of care technology for cost effective lung cancer screening in developing countries as lung cancer is going to be a major burden in coming years.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- GLOBOCAN 2018. International Agency for Research in Cancer 2018. Available online: https://gco.iarc.fr/
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Paoletti L, Jardin B, Carpenter MJ, et al. Current status of tobacco policy and control. J Thorac Imaging 2012;27:213-9. [Crossref] [PubMed]
- Noronha V, Dikshit R, Raut N, et al. Epidemiology of lung cancer in India: Focus on the differences between non-smokers and smokers: A single-centre experience. Indian J Cancer 2012;49:74-81. [Crossref] [PubMed]
- Krishnamurthy A, Gadigi V, Sagar T, et al. The relevance of "Nonsmoking-associated lung cancer" in India: A single-centre experience. Indian J Cancer 2012;49:82-8. [Crossref] [PubMed]
- Batura-Gabryel H, Foremska-Iciek J. Lung cancer in the elderly-increasing epidemiological problem of 21st century. Rocz Akad Med Bialymst 2005;50 Suppl 1:152-5. [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Alberg AJ, Samet JM. Epidemiology of Lung Cancer. Chest 2003;123:21S-49S. [Crossref] [PubMed]
- Zhou W, Christiani DC. East meets west: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer 2011;30:287-92. [Crossref] [PubMed]
- Zhang H, Cai B. The impact of tobacco on lung health in China. Respirology 2003;8:17-21. [Crossref] [PubMed]
- Kleinerman R, Wang Z, Lubin J, et al. Lung cancer and indoor air pollution in rural china. Ann Epidemiol 2000;10:469. [Crossref] [PubMed]
- Kawaguchi T, Matsumura A, Fukai S, et al. Japanese ethnicity compared with Caucasian ethnicity and never smoking status are independent favorable prognostic factors for overall survival in non-small cell lung cancer: a collaborative epidemiologic study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry databases. J Thorac Oncol 2010;5:1001-10. [Crossref] [PubMed]
- Grassi MF, Dos Santos NP, Lírio M, et al. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect Dis 2016;16:491. [Crossref] [PubMed]
- Gao L, Li X, Liu J, et al. Incidence of active tuberculosis in individuals with latent tuberculosis infection in rural China: follow-up results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2017;17:1053-61. [Crossref] [PubMed]
- Goyal V, Kadam V, Narang P, et al. Prevalence of drug-resistant pulmonary tuberculosis in India: systematic review and meta-analysis. BMC Public Health 2017;17:817. [Crossref] [PubMed]
- Hannan A. Misdiagnosis of Cancer as Tuberculosis in Low- to Middle-Income Countries: A Tip of the Iceberg! J Glob Oncol 2016;2:244-5. [Crossref] [PubMed]
- Vaghasiya K, Sharma A, Verma R. Misdiagnosis Murder: Disguised TB or Lung Cancer? Pul Res Respir Med 2016;3:e5-6.
- Singh VK, Chandra S, Kumar S, et al. A Common Medical Error: Lung Cancer Misdiagnosed as Sputum Negative Tuberculosis. Asian Pac J Cancer Prev 2009;10:335-8. [PubMed]
- Bhatt M, Kant S, Bhaskar R. Pulmonary tuberculosis as differential diagnosis of lung cancer. South Asian J Cancer 2012;1:36. [Crossref] [PubMed]
- Sankaranarayanan R. Screening for cancer in low- and middle-income countries. Ann Glob Health 2014;80:412-7. [Crossref] [PubMed]
- Verghese C, Redko C, Fink B. Screening for Lung Cancer Has Limited Effectiveness Globally and Distracts From Much Needed Efforts to Reduce the Critical Worldwide Prevalence of Smoking and Related Morbidity and Mortality. J Glob Oncol 2018;4: JGO.2017.1700016.
- Raez LE, Nogueira A, Santos ES, et al. Challenges in Lung Cancer Screening in Latin America. J Glob Oncol 2018;4:1-10. [Crossref] [PubMed]
- Jones SB. Cancer in the developing world: a call to action. BMJ 1999;319:505-8. [Crossref] [PubMed]
- Notani P, Sanghavi LD. A retrospective study of lung cancer in Bombay. Br J Cancer 1974;29:477-82. [Crossref] [PubMed]
- Behera D, Balamugesh T. Indoor air pollution as a risk factorfor lung cancer in women. J Assoc Physicians India 2005;53:190-2. [PubMed]
- Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, et al. The incidence and mortality of lung cancer and their relationship to development in Asia. Transl Lung Cancer Res 2015;4:763-74. [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Asthana S, Patil RS, Labani S. Tobacco-related cancers in India: A review of incidence reported from population-based cancer registries. Indian J Med Paediatr Oncol 2016;37:152-7. [Crossref] [PubMed]
- Mohan S, Asthana S, Labani S, et al. Cancer trends in India: A review of population-based cancer registries (2005-2014). Indian J Public Health 2018;62:221-3. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev 2014;23:953-66. [Crossref] [PubMed]
- Bello B, Fadahun O, Kielkowski D, et al. Trends in lung cancer mortality in South Africa: 1995-2006. BMC Public Health 2011;11:209. [Crossref] [PubMed]
- Sisti J, Boffetta P. What proportion of lung cancer in never-smokers can be attributed to known risk factors? Int J Cancer 2012;131:265-75. [Crossref] [PubMed]
- Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033-4. [Crossref] [PubMed]
- Mu L, Liu L, Niu R, et al. Indoor Air Pollution and Risk of Lung Cancer among Chinese Female Non-Smokers. Cancer Causes Control 2013;24:439-50. [Crossref] [PubMed]
- Operational Framework Management of Common Cancers. National Institute of Cancer Prevention and Health. Indian Council of Medical Research. Available online: http://nicpr.in/nicprold/images/PDF/Operational_Framework_Management_of_Common_Cancers.pdf
- Bunn PA. Worldwide Overview of the Current Status of Lung Cancer Diagnosis and Treatment. Arch Pathol Lab Med 2012;136:1478-81. [Crossref] [PubMed]
- Brett GZ. The value of lung cancer detection by six-monthly chest radiographs. Thorax 1968;23:414-20. [Crossref] [PubMed]
- Berlin NI, Buncher CR, Fontana RS, et al. The National Cancer Institute Cooperative Early Lung Cancer Detection Program. Results of the initial screen (prevalence). Early lung cancer detection: Introduction. Am Rev Respir Dis 1984;130:545-9. [PubMed]
- Tockman MS. Survival and Mortality from Lung Cancer in a Screened Population. Chest 1986;89:324S-325S. [Crossref]
- Melamed MR, Flehinger BJ, Zaman MB, et al. Screening for Early Lung Cancer. Chest 1984;86:44-53. [Crossref] [PubMed]
- Fontana RS, Sanderson DR, Woolner LB, et al. Lung cancer screening: the Mayo program. J Occup Med 1986;28:746-50. [Crossref] [PubMed]
- Fontana RS, Sanderson DR, Woolner LB, et al. Screening for lung cancer. A critique of the Mayo Lung Project. Cancer 1991;67:1155-64. [Crossref] [PubMed]
- Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. [Crossref] [PubMed]
- Kubík A, Polák J. Lung Cancer detection. Results of a randomized prospective study in Czechoslovakia. Cancer 1986;57:2427-37. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- De Koning H, Van Der Aalst C, Ten Haaf K, et al. Effects of volume CT lung cancer screening: Mortality results of the NELSON randomized-controlled population based trial 2018 World Conference on Lung Cancer. Abstract PL02.05. Presented September 25, 2018.
- McWilliams AM, Mayo JR, Ahn MI, et al. Lung cancer screening using multi-slice thin-section computed tomography and autofluorescence bronchoscopy. J Thorac Oncol 2006;1:61-8. [Crossref] [PubMed]
- Blanchon T, Bréchot JM, Grenier PA, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50-8. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798-802. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized Study on Early Detection of Lung Cancer with MSCT in Germany: Results of the First 3 Years of Follow-up After Randomization. J Thorac Oncol 2015;10:890-6. [Crossref] [PubMed]
- Chong S, Lee KS, Chung MJ, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci 2005;20:402-8. [Crossref] [PubMed]
- Chen CY, Chen CH, Shen TC, et al. Lung cancer screening with low-dose computed tomography: Experiences from a tertiary hospital in Taiwan. J Formos Med Assoc 2016;115:163-70. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman F, et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med 2015;191:1166-75. [Crossref] [PubMed]
- Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72:825-31. [Crossref] [PubMed]
- Hanna N. Survival of Patients with Stage I Lung Cancer Detected on CT Screening. Yearbook Med 2008;2008:175-7. [Crossref]
- Abraham J. Reduced lung cancer mortality with low-dose computed tomographic screening. Community Oncol 2011;8:441-2. [Crossref]
- Chiles C. Lung Cancer Screening with Low Dose CT. Radiol Clin North Am 2014;52:27-46. [Crossref] [PubMed]
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. [Crossref] [PubMed]
- dos Santos RS, Franceschini JP, Chate RC, et al. Do Current Lung Cancer Screening Guidelines Apply for Populations With High Prevalence of Granulomatous Disease? Results From the First Brazilian Lung Cancer Screening Trial (BRELT1). Ann Thorac Surg 2016;101:481-6; discussion 487-8. [Crossref] [PubMed]
- de Sá VK, Coelho J, Capelozzi V, et al. Lung cancer in Brazil: epidemiology and treatment challenges. Lung Cancer 2016;7:141-8. [PubMed]
- Hu P, Dai M, Shi J, et al. The feasibility study of a randomized cancer screening trial in China. Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20; New Orleans, LA. Philadelphia (PA): AACR; Cancer Res 2016;76: Abstract nr 1795.
- Hammen I. Tuberculosis mimicking lung cancer. Respir Med Case Rep 2015;16:45-47. [Crossref] [PubMed]
- Cukic V. The Association Between Lung Carcinoma and Tuberculosis. Medical Archives 2017;71:212. [Crossref] [PubMed]
- Keikha M, Esfahani BN. The Relationship between Tuberculosis and Lung Cancer. Adv Biomed Res 2018;7:58. [Crossref] [PubMed]
- Tuberculosis country profile. WHO. Available online: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=IN&LAN=EN&outtype=html
- Wang Z, Han W, Zhang W, et al. Mortality outcomes of low-dose computed tomography screening for lung cancer in urban China: a decision analysis and implications for practice. Chin J Cancer 2017;36. [Crossref] [PubMed]
- Brown MS, Lo P, Goldin J, et al. Toward clinically usable CAD for lung cancer screening with computed tomography. Eur Radiol 2014;24:2719-28. [Crossref] [PubMed]
- Gierada DS, Pinsky P, Nath H, et al. Projected Outcomes Using Different Nodule Sizes to Define a Positive CT Lung Cancer Screening Examination. JNCI 2014;106. [Crossref] [PubMed]
- de Koning HJ, Meza R, Plevritis S, et al. Benefits and Harms of Computed Tomography Lung Cancer Screening Strategies: A Comparative Modeling Study for the U.S. Preventive Services Task Force. Ann Int Med 2014;160:311. [Crossref] [PubMed]
- Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ 2004;328:1470. [Crossref] [PubMed]
- Manser RL. Screening for lung cancer: a systematic review and meta-analysis of controlled trials. Thorax 2003;58:784-9. [Crossref] [PubMed]
- Flehinger BJ, Melamed MR. Current status of screening for lung cancer. Chest Surg Clin N Am 1994;4:1-15. [PubMed]
- Fink C, Hallscheidt PJ, Noeldge G, et al. Clinical comparative study with a largearea amorphous silicon flat-panel detector: image quality and visibility of anatomic structures on chest radiography. AJR Am J Roentgenol 2002;178:481-6. [Crossref] [PubMed]
- Garmer M, Hennigs SP, Jäger HJ, et al. Digital radiography versus conventional radiography in chest imaging: diagnostic performance of a large-area silicon flat-panel detector in a clinical CT-controlled study. AJR Am J Roentgenol 2000;174:75-80. [Crossref] [PubMed]
- Redlich U, Hoeschen C, Effenberger O, et al. Comparison of four digital and one conventional radiographic image systems for the chest in a patient study with subsequent system optimization. Rofo 2005;177:272-8. [Crossref] [PubMed]
- Hennigs SP, Garmer M, Jaeger HJ, et al. Digital chest radiography with a large-area flat-panel silicon X-ray detector: clinical comparison with conventional radiography. Eur Radiol 2001;11:1688-96. [Crossref] [PubMed]
- Kobayashi T, Xu XW, MacMahon H, et al. Effect of a computer-aided diagnosis scheme on radiologists' performance in detection of lung nodules on radiographs. Radiology 1996;199:843-8. [Crossref] [PubMed]
- Kido S, Ikozoe J, Naito H, et al. Clinical evaluation of pulmonary nodules with single-exposure dual-energy subtraction chest radiography with an iterative noise-reduction algorithm. Radiology 1995;194:407-12. [Crossref] [PubMed]
- MacMahon H, Engelmann RM, Behlen FM, et al. Computer-aided diagnosis of pulmonary nodules: results of a large-scale observer test. Radiology 1999;213:723-6. [Crossref] [PubMed]
- Austin JH, Romney B, Goldsmith L. Missed bronchogenic carcinoma: radiographic findings in 27 patients with a potentially resectable lesion evident in retrospect. Radiology 1992;182:115-22. [Crossref] [PubMed]
- de Hoop B, Schaefer-Prokop C, Gietema H, et al. Screening for Lung Cancer with Digital Chest Radiography: Sensitivity and Number of Secondary Work-up CT Examinations. Radiology 2010;255:629-37. [Crossref] [PubMed]
- van Walbeek C. Recent trends in smoking prevalence in South Africa- some evidence from AMPS data. S Afr Med J 2002;92:468-72. [PubMed]
- Legresley E, Lee K, Muggli ME, et al. British American tobacco and the "insidious impact of illicit trade in cigarettes across Africa. Tob Control 2008;17:339-46. [Crossref] [PubMed]
- Nsimba SE, Sussman S. Tobacco advertisements and promotion industry on smoking in Tanzania: a review of negative public health implications for current and future generations. Tob Induc Dis 2006;3:41. [Crossref] [PubMed]
- Tumwine J. Implementation of the framework convention on tobacco control in Africa: current status of legislation. Int J Environ Res Public Health 2011;8:4312-31. [Crossref] [PubMed]
- Warren CW, Lea V, Lee J, et al. Change in tobacco use among 13-15 year olds between 1999 and 2008: findings from the Global Youth Tobacco Survey. Glob Health Promot 2009;16:38-90. [Crossref] [PubMed]
- Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA 2005;294:1505-10. [Crossref] [PubMed]
- . Available online: https://clinicaltrials.gov/ct2/homeUS National Library of Medicine.
- Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593-7. [Crossref] [PubMed]
- Bastarrika G, García-Velloso MJ, Lozano MD, et al. Early lung cancer detection using spiral computed tomography and positron emission tomography. Am J Respir Crit Care Med 2005;171:1378-83. [Crossref] [PubMed]
- McWilliams A, Mayo J, MacDonald S, et al. Lung cancer screening: a different paradigm. Am J Respir Crit Care Med 2003;168:1167-73. [Crossref] [PubMed]
- Belinsky SA, Liechty KC, Gentry FD, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res 2006;66:3338-44. [Crossref] [PubMed]
- Kersting M, Friedl C, Kraus A, et al. Differential frequencies of p16(INK4a) promoter hypermethylation, p53 mutation, and K-ras mutation in exfoliative material mark the development of lung cancer in symptomatic chronic smokers. J Clin Oncol 2000;18:3221-9. [Crossref] [PubMed]
- Ahrendt SA, Chow JT, Xu LH, et al. Molecular detection of tumor cells in bronchoalveolar lavage fluid from patients with early stage lung cancer. J Natl Cancer Inst 1999;91:332-9. [Crossref] [PubMed]
- Baryshnikova E, Destro A, Infante MV, et al. Molecular alterations in spontaneous sputum of cancer-free heavy smokers: results from a large screening program. Clin Cancer Res 2008;14:1913-9. [Crossref] [PubMed]
- Targowski T, Jahnz-Rozyk K. Diagnostic and prognostic value of telomerase assay in ling cancer. Arch Med Sci 2008;4:353-7.
- Lam S, Kennedy T, Unger M, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest 1998;113:696-702. [Crossref] [PubMed]
- Häussinger K, Becker H, Stanzel F, et al. Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomised controlled multicentre trial. Thorax 2005;60:496-503. [Crossref] [PubMed]
- Hirsch FR, Prindiville SA, Miller YE, et al. Fluorescence versus white light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst 2001;93:1385-91. [Crossref] [PubMed]
- Ernst A, Simoff MJ, Mathur PN, et al. D-light autofluorescence in the detection of premalignant airway changes: a multicenter trial. J Bronchology Interv Pulmonol 2005;12:133-8.
- Edell E, Lam S, Pass H, et al. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy - an international, multicenter clinical trial. J Thorac Oncol 2009;4:49-54. [Crossref] [PubMed]
- Chiyo M, Shibuya K, Hoshino H, et al. Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer 2005;48:307-13. [Crossref] [PubMed]
- Phillips M, Gleeson K, Hughes JM, et al. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet 1999;353:1930-3. [Crossref] [PubMed]
- Phillips M, Cataneo RN, Cummin AR, et al. Detection of lung cancer with volatile markers in the breath. Chest 2003;123:2115-23. [Crossref] [PubMed]
- Machado RF, Laskowski D, Deffenderfer O, et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am J Respir Crit Care Med 2005;171:1286-91. [Crossref] [PubMed]
- Chapman EA, Thomas PS, Stone E, et al. A breath test for malignant mesothelioma using an electronic nose. Eur Respir J 2012;40:448-54. [Crossref] [PubMed]
- D’Amico A, Pennazza G, Santonico M, et al. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010;68:170-6. [Crossref] [PubMed]
- Di Natale C, Macagnano A, Martinelli E, et al. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens Bioelectron 2003;18:1209-18. [Crossref] [PubMed]
- Dragonieri S, Annema JT, Schot R, et al. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009;64:166-70. [Crossref] [PubMed]
- Shlomi D, Abud M, Liron O. Detection of lung cancer and EGFR mutation by electronic nose system. J Thorac Oncol 2017;12:1544-1. [Crossref] [PubMed]
- McWilliams A, Beigi P, Srinidhi A, et al. Sex and smoking status effects on the early detection of early lung cancer in high-risk smokers using an electronic nose. IEEE Trans Biomed Eng 2015;62:2044-54. [Crossref] [PubMed]
- van de Goor R, van Hooren M, Dingemans AM, et al. Training and Validating a Portable Electronic Nose for Lung Cancer Screening. J Thorac Oncol 2018;13:676-81. [Crossref] [PubMed]
- Petty RD, Nicolson MC, Kerr KM, et al. Gene expression profiling in non-small cell lung cancer: from molecular mechanisms to clinical application. Clin Cancer Res 2004;10:3237-48. [Crossref] [PubMed]
- Rahman SM, Shyr Y, Yildiz PB, et al. Proteomic patterns of preinvasive bronchial lesions. Am J Respir Crit Care Med 2005;172:1556-62. [Crossref] [PubMed]
- Phillips M. Method for the collection and assay of volatile organic compounds in breath. Anal Biochem. 1997;247:272-8. [Crossref] [PubMed]
- Nardi-Agmon I, Peled N. Exhaled breath analysis for the early detection of lung cancer: recent developments and future prospects. Lung Cancer (Auckl) 2017;8:31-8. [Crossref] [PubMed]
- Nakhleh MK, Amal H, Jeries R, et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017;11:112-25. [Crossref] [PubMed]
- Field JK, Smith R, Aberle D, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 Report. J Thorac Oncol 2012;7:10-9. [Crossref] [PubMed]
- IASLC CT Screening Workshop 2011 Participants, Field JK, Smith RA, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol 2012;7:10-9.
- Pinsky PF. Lung cancer screening with low-dose CT: a world-wide view. Transl Lung Cancer Res 2018;7:234-42. [Crossref] [PubMed]
- Japan Radiologic Society. The Japanese Imaging Guideline, 2013. Available online: http://www.radiology.jp/english/guideline.html
- Jazieh AR, AlGhamdi M, AlGhanem S, et al. Saudi lung cancer prevention and screening guidelines. Ann Thorac Med 2018;13:198. [Crossref] [PubMed]
- Canadian Task Force on Preventive Health Care. Lung Cancer (2016). Available online: https://canadiantaskforce.ca/guidelines/published-guidelines/lung-cancer/
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- American Academy of Family Physicians. Lung cancer clinical recommendations. Available online: http://www.aafp.org/patient-care/clinical-recommendations/all/lung-cancer.html
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [Crossref] [PubMed]
- Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2016;66:96-114. [Crossref] [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-92S.
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- American Lung Association. Providing guidance on lung cancer screening to patients and physicians. An update from the American Lung Association Screening Committee. April 30, 2015. Available online: http://www.lung.org/assets/documents/lung-cancer/lung-cancer-screening-report.pdf
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- Moyer VA. US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]


