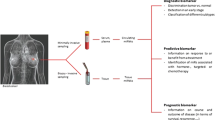
Abstract
MicroRNAs (miRs) are small single-stranded RNA molecules, which function as key negative regulators of post-transcriptional modulation in almost all biological processes. Abnormal expression of microRNAs has been observed in various types of cancer including breast cancer. Great efforts have been made to identify an association between microRNA expression profiles and breast cancer, and to understand the functional role and molecular mechanism of aberrant-expressed microRNAs. As research progressed, ‘oncogenic microRNAs’ and ‘tumor suppressive microRNAs’ became a focus of interest. The potential of candidate microRNAs from both intercellular (tissue) and extracellular (serum) sources for clinical diagnosis and prognosis was revealed, and treatments involving microRNA achieved some amazing curative effects in cancer disease models. In this review, advances from the most recent studies of microRNAs in one of the most common cancers, breast cancer, are highlighted, especially the functions of specifically selected microRNAs. We also assess the potential value of these microRNAs as diagnostic and prognostic markers, and discuss the possible development of microRNA-based therapies.
概要
搜集已报道的在乳腺癌发生发展过程中有重要作用的microRNA信息, 结合相关肿瘤模型的实验数据, 评估microRNA在乳腺癌诊断和治疗方面的应用前景。 以“癌基因”和“癌抑制基因”为分类依据, 全面地总结归纳乳腺癌相关microRNA的功能和作用机制, 进一步从临床诊断标记物和治疗靶点的层面分析microRNA的潜在临床应用价值。 表1和2总结了microRNA参与乳腺癌发展过程的具体事件及其调控的靶基因。 同时, 本文还深入探讨microRNA作为临床诊断标记物及治疗靶点的可行性, 揭示已有研究的不足之处, 为今后的相关工作方向提供一些建议。
Similar content being viewed by others
1 Introduction
Cancer has long been the gravest challenge to human health, which not only reduces the quality of life, but also increases mortality. Breast cancer is the most common malignancy and the leading cause of death by cancer in women, with more than 500 000 cases globally per year (Iorio and Croce, 2009; Zoon et al., 2009). Under this complex neoplasma, the scenario consists of tumor initiation and growth, metastasis and invasion, angiogenesis and possible relapse. All of these stages may be affected by changes in genetic information and maintenance of the tumor microenvironment (TME) (Al-Hajj et al., 2003; Brabletz et al., 2005; Sica et al., 2008). As breast cancer is one kind of highly-heterogeneous tumor, a number of expression signatures have been developed to classify its molecular subtypes. According to the St. Gallen symposium, five breast tumor subtypes are well defined: luminal A, luminal B, basal-like, HER2+ (human epidermal growth factor receptor 2 positive), and normal breast-like. ER+ (estrogen receptor positive) is the main feature of the luminal A/B subtypes, and the others are ER- subtypes (Sorlie et al., 2001; Bertucci et al., 2005; Blenkiron et al., 2007).
Recently, cancer stem cells (CSCs) have received more attention, especially in breast cancer. Although the CSC theory was always accompanied by scepticism and intense debate, numerous evidence has proved the existence in tumors of this minority subpopulation with amazing tumor-initiating powers. In some cases, tumor-initiating cells are also called CSCs to represent their CSC characteristics. The issues about how CSCs come into being, why they have the ability to initiate and drive tumor growth, and how they can be resistant to therapeutics remain confused (Shipitsin and Polyak, 2008; Valent et al., 2012; Kreso and Dick, 2014). The activation of epithelial-to-mesenchymal transition (EMT), which plays a crucial role in cell-type interconversions during the normal development of organogenesis, can induce the acquisition of mesenchymal traits and the expression of stemness markers in cancer cells. Understanding this key process in tumor initiation and metastasis may explain how CSCs form and suggest a possible avenue for their therapeutic manipulation (Karnoub et al., 2007; Mani et al., 2008; Scheel et al., 2011). To improve the ability for diagnosis and treatment and reduce the danger of cancer, many scientists have explored the etiological mechanisms at the cellular or molecular level. Increasing evidence supports the view that a change in epigenetic modifications, microenvironment or specific-gene expression occurs in many types of cancer progression (Calin and Croce, 2006; Iorio and Croce, 2009; Hermeking, 2012). Some of the small aberrant effective molecules, like microRNAs, have the potential to be markers for diagnosis and prognosis, or serve as effective targets for therapeutic interventions (Heneghan et al., 2010; Jansson and Lund, 2012).
MicroRNAs are a group of small molecules that are highly conserved evolutionarily and encoded by about 1% of the genome in most species (Bartel, 2004). Since the discovery of lin-4 (Lee et al., 1993), the exploration of this kind of single-stranded RNA has been the focus of much attention. With the help of polymerase II, a primary microRNA is transcribed from a coding-gene intron or the intergenic region like other regular mRNA. Following further processing with a series of enzymes or functional proteins, such as Drosha, Exportin 5, and Dicer, the mature microRNA can be spliced from its precursor (Kim, 2005). Although existing as mini bodies of only about 22 nt, microRNAs play an important role in post-transcriptional regulation by binding to their targets in an incomplete base-pairing manner, particularly in mRNA 3′ untranslated regions, causing translation suppression or mRNA degradation (Bartel, 2004; O’Hara et al., 2009). Many studies have proved that microRNAs are highly specific in their expression in different tissues and development stages (Lagos-Quintana et al., 2002), and play an essential role in diverse biological events, such as cell proliferation, differentiation, and apoptosis (Croce and Calin, 2005). Recent data have demonstrated that microRNAs located in one cluster can work together in mapping a regulatory network by binding their target to fulfill the same mission, which enhances their function in both physiological and pathological processes (Tanzer and Stadler, 2004).
It is well known that some microRNAs are deregulated in numerous kinds of disease, including breast cancer. Abnormal microRNA expression involved in the cancer process provides a suitable entry point to explore its functional role in cancer progression. With the rapid development of sequencing and molecular biology techniques, amplified or decreased microRNA can easily be detected and functional microRNAs readily identified. Up-regulated ‘oncogenic microRNAs’ inhibit the expression of potential tumor suppressive genes, while down-regulated ‘tumor suppressive microRNAs’ cause the augmentation of downstream signal pathways responsible for tumor development. Both can promote the development and progression of cancer. These two kinds of population may participate in pathogenic pathways by targeting the same or different molecules within a complicated regulatory network. Different cancers may share common aberrant microRNAs, although cancerspecific microRNAs are the major population. In this review, we focus on recent evidence about the role of oncogenic and tumor suppressive microRNAs in breast cancer and discuss their applications to clinical approaches, especially their potential to be diagnostic and prognostic markers and therapeutic targets (Iorio et al., 2005; Iorio and Croce, 2009; Andorfer et al., 2011; Melo and Esteller, 2011).
2 Oncogenic microRNAs in breast cancer
2.1 miR-21
miR-21 is one of the most well-known tumorpromoting microRNAs in many species of cancer, whose expression is dramatically up-regulated in breast cancer. Numerous studies have confirmed its association with advanced clinical stages of cancer, tumor metastasis, and poor prognosis. Several genes associated with tumor growth and metastasis, such as tumor suppressor tropomyosin 1 (TPM1), programmed cell death 4 (PDCD4), TIMP metallopeptidase inhibitor 3 (TIMP3), and phosphatase and tensin homolog (PTEN), have been proved to be targeted by miR-21 (Meng et al., 2007; Zhu et al., 2007; Frankel et al., 2008; Qi et al., 2013). The overexpression of miR-21 in tumor cells could alleviate their function in ‘suppressing’ cell apoptosis and death. By regulating target genes and modulating the following downstream pathway, miR-21 aberrant expression results in tumor metastasis and invasion. Knocking down its expression could attenuate its contribution in the cancer process. Recent studies also found high levels of miR-21 in breast cancer patient serum (Wang et al., 2010; Asaga et al., 2011). Its increased expression can also be detected in a variety of other malignancies including glioblastoma, ovarian cancer, lung cancer, and colorectal cancer (Chan et al., 2005; Yanaihara et al., 2006; Iorio et al., 2007; Schee et al., 2012; Wang and Zhang, 2012), indicating that miR-21 may have diagnostic and prognostic values.
2.2 miR-155
miR-155 is frequently up-regulated in breast tumor tissue. Its ectopic expression influences tumor cell survival and chemosensitivity through down-regulating forkhead box O3 (FOXO3a), whereas knocking down the expression of miR-155 can enhance cell chemosensitivity and mediate apoptosis (Kong et al., 2010). Other work supports the suggestion that miR-155 plays its oncogenic role by targeting suppressor of cytokine signaling 1 (SOCS1). Inflammation stimuli can up-regulate miR-155 in breast cancer cells, leading to the activation of STAT3 (signal transducer and activator of transcription 3). This indicates a possible functional link between inflammation and cancer mediated by miR-155 (Jiang et al., 2010). In addition, caspase-3, a potent suppressor of apoptosis, has been reported to be a target of miR-155 (Ovcharenko et al., 2007).
2.3 miR-182
EMT is the initial event of breast tumor-initial cell (BT-IC)-associated metastasis. β-Catenin, a key regulator in EMT, can bind to the promoter of miR-182 and positively regulate miR-182 expression in breast cancer cells. miR-182 is also overexpressed in human breast tumor tissues. By repressing its target gene reversion-inducing-cysteine-rich protein with kazal motifs (RECK), ectopic expression of miR-182 induces matrix metallopeptidase 9 (MMP-9) activity, cell invasion, and colony formation, and further increases tumorigenicity and invasiveness (Chiang et al., 2013). Other data also demonstrate that both MIM (missing in metastasis, which activates the Ras homolog family member A) and FOXO1 (forkhead box protein O1, a putative tumor suppressor transcription factor) are the targets of miR-182 and are involved in its promotion of breast cancer metastasis (Guttilla and White, 2009; Lei et al., 2014).
2.4 miR-10b
In metastatic breast cancer, miR-10 has been confirmed as a ‘metastasis miR’, a crucial downstream effector of twist-related protein 1 (TWIST1) (Ma et al., 2007). By numbing the homeobox D10 (HOXD10) tumor suppressor signaling pathway and regulating T lymphoma invasion and metastasis 1 (Tiam1)-mediated Rac activation, miR-10b makes a significant contribution to the invasion and migration of breast cancer cells (Moriarty et al., 2010; Haque et al., 2011). The expression level of miR-10b is positively correlated with pathological grading, clinical staging, and lymph node metastasis. A novel E-cadherin-related mechanism has recently been shown to be involved in miR-10b modulation of breast cancer metastasis (Liu et al., 2012).
2.5 miR-27a
miR-27a, which is overexpressed in breast cancer cells, promotes cell viability by promoting cell cycle traverse and inhibiting cell death by targeting FOXO1 (Guttilla and White, 2009). Tang et al. (2014) revealed that increased miR-27a expression is associated with reduced expression of ZBTB10 (zinc finger and BTB domain containing 10), which has been validated as a target of miR-27a. This results in the promotion of angiogenesis by mediating breast CSC properties. Moreover, the zinc finger protein ZBTB10 gene, as a putative specificity protein (Sp) repressor, which contributes to cancer angiogenic ability, helps miR-27b fulfill its oncogenic function (Mertens-Talcott et al., 2007).
2.6 miR-9
Tumor cell motility and invasiveness is an important reference point of tumor metastasis. miR-9, which is expressed more highly in breast tumor tissue than in normal tissue, promotes the oncogenic process by negatively regulating E-cadherin. Also, miR-9 indirectly induces the expression of vascular endothelial growth factor (VEGF) and contributes to angiogenesis at the same time, promoting cancer development. Thus, miR-9 is an example of a signal microRNA that may regulate one process with different targets (Ma et al., 2010a).
3 Tumor suppressive microRNAs in breast cancer
3.1 let-7 family
The let-7 families are the most ancient and conserved microRNAs. They were discovered in Caenorhabditis elegans (Johnson et al., 2003). They function as well-recognized tumor suppressive microRNAs in a heterochronic pathway that is necessary for cancers, including breast cancer, to undergo correct initiation, differentiation, and metastasis at the appropriate time (Nimmo and Slack, 2009). In the initial stage of a breast tumor, let-7a is involved in BT-IC stem cell-like activities by silencing its target genes H-Ras (transforming protein p21) and HMGA2 (high-mobility group AT-hook 2). Overexpression of let-7 effectively delays the development of breast cancer (Yu et al., 2007). Sun et al. (2012) claimed that down-regulation of let-7b/c in breast CSCs caused let-7b/c to lose its ability to restrain Ras mRNA, resulting in the activation of p-Ras and p-ErK. This revealed an important role of let-7 in maintaining breast cancer cell stemness. let-7b is often down-regulated in lymph node metastases of breast cancer cells. By silencing four target genes related to the actin cytoskeleton pathway, PAK1 (serine/threonine-protein kinase 1), DIAPH2 (also known as protein diaphanous homolog 2), RDX (radixin), and ITGB8 (integrin β-8), breast migration and invasion are greatly inhibited (Hu et al., 2013).
3.2 miR-145
miR-145 exerts its tumor suppressive function by silencing different target genes in stage-specific events. Insulin receptor substrate-1 (IRS-1), which is necessary for BT-IC differentiation, has been shown to be a direct target of miR-145 (Rubin et al., 2007). ER-α is reported to be negatively regulated by miR-145 at two complementary sites, thereby mediating breast cancer cell growth (Spizzo et al., 2010). Rhotekin (RTKN) protein expression can be significantly reduced by miR-145, resulting in inhibition of cell growth and induced apoptosis (Wang et al., 2009). miR-145 also can directly target mucin 1 (MUC1), a metastasis gene, leading to a reduction of β-catenin and cadherin 11 and giving rise to suppressed cell invasion and lung metastasis (Sachdeva and Mo, 2010). Additionally, miR-145 was proved to inhibit breast cancer cell EMT by blocking the expression of octamerbinding transcription factor 4 (Oct4) (Hu et al., 2012).
3.3 miR-200 family
miR-200 families have a moderating effect on controlling the transition between CSC-like and nonstem-cell-like phenotypes. Under stem-like phenotypes, through epigenetic modifications, the miR-200b-200a-429 cluster was silenced, followed by the repressed expression of the miR-200c-141 cluster (Lim et al., 2013). miR-200 families also exert their effects in distinct metastatic stages in a moesin-dependent manner. miR-200b can regulate tumor cell plasticity and metastasis as a tumor suppressor (Li X. et al., 2013). However, Dykxhoorn et al. (2009) found that while miR-200b/c promotes mesenchymal-to-epithelial cell transition (MET) by inhibiting ZEB2 (zinc finger E-box-binding homeobox 2) expression, it also promotes macroscopic metastases in breast cancer cell lines. Therefore, the whole story of the miR-200 family needs further investigation.
3.4 miR-205
miR-205, which is significantly down-regulated in human breast tumor tissue, negatively regulates EMT by targeting ZEB1 and ZEB2. It is also expressed at low levels in mesenchymal breast cancer cell lines and triple negative breast cancers (Gregory et al., 2008; Radojicic et al., 2011). HER2 is a hallmark of aggressive breast tumors, and is driven largely through phosphorylation of HER3. By targeting HER3, miR-205 could interfere with the HER receptor family-mediated proliferative pathway (Iorio et al., 2009). In in vitro experiments, ectopic expression of miR-205 in breast cancer cells suppressed cell proliferation, growth, and invasion by directly targeting HER3 and VEGF-A (Wu et al., 2009; Wang et al., 2013). Most recently, Chao et al. (2014) indicated that miR-205 secreted from the tumor stroma can help promote a cancer-associated stem cell phenotype, suggesting that targeting-miR-205 may have a potential therapeutic value.
3.5 miR-335
Deletion or epigenetic silencing of miR-335 is a common event in breast cancer metastasis (Png et al., 2011). Tavazoie et al. (2008) reported that miR-335 can repress breast cancer migration and invasion through targeting SRY-related HMG-box 4 (SOX4) and tenascin C. By simultaneously regulating the known breast cancer 1 (BRCA1) activators, ER-α, IGF1 and Sp1, and the repressor ID4, miR-335 exerts its tumor-suppressive function by decreasing cell viability and inducing apoptosis (Heyn et al., 2011).
3.6 miR-19a
The TME exerts a dominant role in the cancer process. Tumor associated-macrophages (TAMs) are the major components of the TME comprising about 40%. The transition of TAMs from a pro-immune (M1-like) phenotype to an immune-suppressive (M2-like) phenotype is one of the hallmarks of malignancy (Mukhtar et al., 2011). Down-regulated miR-19a in TAMs, induced by the TME, is able to maintain the phenotype of TAMs by targeting Fos-related antigen 1 (Fra-1) and other genes in its downstream signaling pathway. The reintroduction of miR-19a to this kind of macrophage can promote transformation of M1 to M2, resulting in the enhancement of migration and invasion of breast cancer cells (Yang et al., 2014).
4 Other breast cancer-associated microRNAs
5 MicroRNAs as possible markers for diagnosis and prognosis in breast cancer
With the rapid development of gene microarrays and experimental technologies, an increasing and encouraging number of studies are demonstrating the contribution of microRNAs to the pathogenesis and progression of breast cancer. Indeed, abnormal microRNA expression patterns are closely related to specific tumor stages, lymph node metastasis, poor survival, disease outcomes and responses to specific therapies in many types of cancer. Apart from those with traditional intercellular functions, diverse forms of microRNA have been found in the past decade: serum, saliva, urine, and milk all contain microRNAs, which are packaged by microvesicles or exosomes, or exist as compounds with protective modifications (Gilad et al., 2008; Mitchell et al., 2008; Park et al., 2009; Chen et al., 2010; Hanke et al., 2010).
Considering that the profiling of microRNAs correlates with biological processes more precisely than gene expression profiling, profiling of microRNAs has been used to diagnose breast cancer at an early stage and determine the prognosis of therapy in breast cancer patients. Blenkiron et al. (2007) first analyzed miRNA expression and genomic changes in human breast cancer. They used the distinct miRNA signatures of different molecular breast tumor subtypes (Luminal A, Luminal B, basal-like, HER2+, and normal-like) for characterization in prognosis. Soon after, Farazi et al. (2011) accomplished deep sequencing of microRNA in breast tumors and showed that the microRNA 17–92 cluster has a high level in triple-negative breast carcinomas, distinct from other tumor subtypes. Integrated mRNA and microRNA expression profiling in breast cancer brings us more information about microRNAs associated with prognosis. High levels of miR-767-3p, miR-128a, and miR-769-3p are associated with a poor prognosis, the same as miR-27b, miR-144, and/or miR-210 in ER-negative cases (Buffa et al., 2011). let-7 family members are down-modulated in metastasis lymph node or breast cancer samples with a high proliferation index, suggesting that a deficiency of let-7 family microRNAs is associated with a poor prognosis (Iorio et al., 2005). Also, the miR-106b-25 cluster is available to predict relapse more quickly (Smith et al., 2012). miR-181a (Taylor et al., 2013) and the miR-221/miR-222 cluster (Chen et al., 2013) are valuable diagnostic and prognostic candidates because of their positive correlation with tumor development. Above all, this indicates that microRNAs have the potential to be diagnostic and prognostic markers. Moreover, several microRNAs have been identified that are specifically deregulated in the blood plasma of breast cancer patients compared to healthy subjects (Cookson et al., 2012; Cuk et al., 2013). The expression of miR-451, miR-21, and miR-16 in the serum of breast cancer patients was amplified compared to that of healthy individuals (Nguyen et al., 2014). MicroRNA aberrant expression has also been quantified in breast cancer patient serum. miR-21, miR-106a, and miR-155 were significantly overexpressed, whereas the expression of miR-126, miR-199a, and miR-335 in tumor specimens was opposite to that of normal specimens (Wang et al., 2010). Interestingly, the above elevated microRNA levels were drastically lowered in postoperative compared with preoperative cases. All of these findings support the suggestion that these circulating microRNAs in serum can serve as diagnostic markers for breast cancer (Wang et al., 2010; Cuk et al., 2013; Ng et al., 2013).
6 Analysis of the prospects and challenges for microRNA-targeted therapy in breast cancer
Despite advances in detection and therapy, breast cancer is still a major challenge for our medical workers. Traditional treatments such as surgery, chemotherapy, and radiotherapy, inevitably have side effects, although they have been undeniably effective. Accompanied by the emerging evidence for the participation of microRNAs in cancer, the potential usefulness of microRNA-based therapy in cancer is now being exploited. By using microRNAs as both targets and tools, microRNA-based therapy has proved to be feasible and efficacious in preclinical models. The inhibition of oncogenic miR-21 reduces tumor development and metastatic potential by way of pro-apoptotic and anti-proliferative effects (Si et al., 2007). Re-introduction of miR-205 improves the responsiveness to tyrosine kinase inhibitors through numbing HER3 in breast cancer cells (Meng et al., 2006). Knocking down miR-34 has a radiosensitizing effect in p53-mutant breast cancer (Weidhaas et al., 2007). Systemic treatment of tumor-bearing mice with miR-10b antagomirs also produced satisfactory curative effects in suppressing breast cancer metastasis (Ma et al., 2010b). Additionally, in ER-positive metastatic breast cancer, let-7 administration has proved to be an effective method against mousemodel breast cancer by regulating apoptosis and CSC differentiation (Barh et al., 2010). There are many impressive cases suggesting that microRNAs may be a viable approach to augment current cancer therapies.
Results to date provide the experimental bases for the use of microRNAs as both targets and tools in anti-cancer therapy, but there are at least three essential issues to address to translate microRNA-based therapy advances from fundamental experiments into medical practice: (1) engineered animal models need to be explored to study cancer-associated microRNAs, fully controlling all their effects on every tumor event; (2) the delivery efficiency of miRNAs/anti-miRNAs in vivo needs to be improved by solving the problems of degradation and instability; (3) the specificity and targeted ability of the delivery system need to be enhanced, avoiding damage to normal tissues. To overcome these issues, modified microRNAs and suitable carriers need ongoing development. Adenovirus (Esquela-Kerscher et al., 2008) and adenoassociated virus (AVV) (Kota et al., 2009), which are used for expressing microRNA, are more useful than synthetic double-stranded hairpins for overcoming the vulnerability of unmodified dsRNAs. To achieve loss-of-function in microRNAs, 2′-O-methyl oligonucleotides (Meister et al., 2004), antagomirs (intravenous administration of cholesterol-conjugated AMOs) (Krützfeldt et al., 2005), locked nucleic acid (LNA)-oligonucleotides (Ørom et al., 2006), and microRNA sponges (microRNA inhibitory transgenes) (Ebert et al., 2007) have been employed to inhibit exogenously introduced microRNAs with high specificity. Also, active small molecule clinical compounds and peptide nucleic acids (PNAs) (Brognara et al., 2012), such as ‘azobenzene’ (Gumireddy et al., 2008), have been tested for their ability to inhibit the expression of specific microRNAs. To improve cellular delivery, the methods for short-interfering RNA (siRNA) or short heteroduplex RNA (shRNA) could also be applied to microRNAs (Dykxhoorn et al., 2006), although excessive shRNA may increase the probability of off-target silencing and elicit nonspecific effects. Furthermore, a highly specific ligandtargeting of liposomal nanoparticles (NPs) (Liao et al., 2011) to solid tumors has been shown to improve tumor selectivity and drug sensitivity in a drug test, by enhancing solid-tumor penetration and uptake of tumor cells. This kind of delivery system eliminates accumulation in normal tissues and circulation, which could also help to avoid systemic toxicity induced of microRNA delivery.
7 Conclusions
In recent decades, increasing efforts have been made to elucidate the molecular mechanisms involved in breast cancer. The results from this work support the crucial role played by dysregulation of specific microRNAs in breast cancer progression. The molecular mechanisms underlying the pathological process mediated by microRNAs have largely been demonstrated. Based on the details discussed above, microRNAs have tremendous potential for clinical diagnosis and prognosis. Defining the functional network connecting microRNAs and their targets will contribute to their usefulness as a therapeutic target. Although there are infinite possibilities for using microRNAs in clinical treatments, some puzzling issues remain to be addressed. For instance, among the numerous deregulated microRNAs in breast cancer, we do not know which is the most representative for each cancer stage. We do not know whether the microRNAs found at high levels in the serum of breast cancer patients are released from the tumor, and what are their major functions. Single microRNAs could target multi-genes, and mRNA might also bind to different microRNAs, so it is possible that one microRNA could participate in various events in both cancer progression and normal tissue development, creating uncertainty in microRNAbased therapy. In the future, under-explored problems such as how to use microRNAs as markers for diagnosis and prognosis, and how to apply miRNA-based therapy in clinics to treat human cancers, may be the biggest challenges to be solved.
Compliance with ethics guidelines
Wei WANG and Yun-ping LUO declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Ahmad, A., Aboukameel, A., Kong, D.J., et al., 2011. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res., 71(9):3400–3409. [doi:10.1158/0008-5472.CAN-10-0965]
Al-Hajj, M., Wicha, M.S., Benito-Hernandez, A., et al., 2003. Prospective identification of tumorigenic breast cancer cells. PNAS, 100(7):3983–3988. [doi:10.1073/pnas.0530291100]
Andorfer, C.A., Necela, B.M., Thompson, E.A., et al., 2011. MicroRNA signatures: clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol. Med., 17(6):313–319. [doi:10.1016/j.molmed.2011.01.006]
Asaga, S., Kuo, C., Nguyen, T., et al., 2011. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin. Chem., 57(1):84–91. [doi:10.1373/clinchem.2010.151845]
Barh, D., Malhotra, R., Ravi, B., et al., 2010. MicroRNA let-7: an emerging next-generation cancer therapeutic. Curr. Oncol., 17(1):70–80. [doi:10.3747/co.v17i1.356]
Bartel, D.P., 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116(2):281–297. [doi:10.1016/S0092-8674(04)00045-5]
Bertucci, F., Finetti, P., Rougemont, J., et al., 2005. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res., 65(6): 2170–2178. [doi:10.1158/0008-5472.CAN-04-4115]
Bhaumik, D., Scott, G.K., Schokrpur, S., et al., 2008. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene, 27(42):5643–5647. [doi:10.1038/onc.2008.171]
Blenkiron, C., Goldstein, L.D., Thorne, N.P., et al., 2007. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol., 8(10):R214. [doi:10.1186/gb-2007-8-10-r214]
Bockhorn, J., Yee, K., Chang, Y.F., et al., 2013. MicroRNA-30c targets cytoskeleton genes involved in breast cancer cell invasion. Breast Cancer Res. Treat., 137(2):373–382. [doi:10.1007/s10549-012-2346-4]
Brabletz, T., Jung, A., Spaderna, S., et al., 2005. Migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer, 5(9):744–749. [doi:10.1038/nrc1694]
Brognara, E., Fabbri, E., Aimi, F., et al., 2012. Peptide nucleic acids targeting miR-221 modulate p27Kip1 expression in breast cancer MDA-MB-231 cells. Int. J. Mol. Med., 30:S59. [doi:10.3892/ijo.2012.1632]
Buffa, F.M., Camps, C., Winchester, L., et al., 2011. MicroRNA-associated progression pathways and potential therapeutic targets identified by integrated mRNA and microRNA expression profiling in breast cancer. Cancer Res., 71(17): 5635–5645. [doi:10.1158/0008-5472.CAN-11-0489]
Cai, J.C., Guan, H.Y., Fang, L.S., et al., 2013. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J. Clin. Invest., 123(2):566–579. [doi:10.1172/Jci65871]
Calin, G.A., Croce, C.M., 2006. MicroRNA signatures in human cancers. Nat. Rev. Cancer, 6(11):857–866. [doi:10.1038/nrc1997]
Cascio, S., D’Andrea, A., Ferla, R., et al., 2010. miR-20b modulates VEGF expression by targeting HIF-1α and STAT3 in MCF-7 breast cancer cells. J. Cell Physiol., 224(1):242–249. [doi:10.1002/Jcp.22126]
Cha, S.T., Chen, P.S., Johansson, G., et al., 2010. MicroRNA-519c suppresses hypoxia-inducible factor-1α expression and tumor angiogenesis. Cancer Res., 70(7):2675–2685. [doi:10.1158/0008-5472.CAN-09-2448]
Chan, J.A., Krichevsky, A.M., Kosik, K.S., 2005. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res., 65(14):6029–6033. [doi:10.1158/0008-5472.CAN-05-0137]
Chao, C.H., Chang, C.C., Wu, M.J., et al., 2014. MicroRNA-205 signaling regulates mammary stem cell fate and tumorigenesis. J. Clin. Invest., 124(7):3093–3106. [doi:10.1172/JCI73351]
Chen, W.X., Hu, Q., Qiu, M.T., et al., 2013. miR-221/222: promising biomarkers for breast cancer. Tumor Biol., 34(3):1361–1370. [doi:10.1007/s13277-013-0750-y]
Chen, X., Gao, C., Li, H.J., et al., 2010. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res., 20(10):1128–1137. [doi:10.1038/cr.2010.80]
Cheng, C.W., Wang, H.W., Chang, C.W., et al., 2012. MicroRNA-30a inhibits cell migration and invasion by downregulating vimentin expression and is a potential prognostic marker in breast cancer. Breast Cancer Res. Treat., 134(3):1081–1093. [doi:10.1007/s10549-012-2034-4]
Chiang, C.H., Hou, M.F., Hung, W.C., 2013. Up-regulation of miR-182 by β-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. BBA-Gen. Subjects, 1830(4):3067–3076. [doi:10.1016/j.bbagen.2013.01.009]
Cittelly, D.M., Das, P.M., Spoelstra, N.S., et al., 2010. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol. Cancer, 9(1):317. [doi:10.1186/1476-4598-9-317]
Cookson, V.J., Bentley, M.A., Hogan, B.V., et al., 2012. Circulating microRNA profiles reflect the presence of breast tumours but not the profiles of microRNAs within the tumours. Cell. Oncol., 35(4):301–308. [doi:10.1007/s13402-012-0089-1]
Croce, C.M., Calin, G.A., 2005. miRNAs, cancer, and stem cell division. Cell, 122(1):6–7. [doi:10.1016/j.cell.2005.06.036]
Cui, W.J., Zhang, S., Shan, C.L., et al., 2013. MicroRNA-133a regulates the cell cycle and proliferation of breast cancer cells by targeting epidermal growth factor receptor through the EGFR/Akt signaling pathway. FEBS J., 280(16):3962–3974. [doi:10.1111/febs.12398]
Cuk, K., Zucknick, M., Heil, J., et al., 2013. Circulating microRNAs in plasma as early detection markers for breast cancer. Int. J. Cancer, 132(7):1602–1612. [doi:10.1002/ijc.27799]
de Souza Rocha Simonini, P., Breiling, A., Gupta, N., et al., 2010. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor α in breast cancer cells. Cancer Res., 70(22): 9175–9184. [doi:10.1158/0008-5472.CAN-10-1318]
Ding, X.M., Park, S.I., McCauley, L.K., et al., 2013. Signaling between transforming growth factor β (TGF-β) and transcription factor SNAI2 represses expression of microRNA miR-203 to promote epithelial-mesenchymal transition and tumor metastasis. J. Biol. Chem., 288(15): 10241–10253. [doi:10.1074/jbc.M112.443655]
Dykxhoorn, D.M., Palliser, D., Lieberman, J., 2006. The silent treatment: siRNAs as small molecule drugs. Gene Therapy, 13(6):541–552. [doi:10.1038/sj.gt.3302703]
Dykxhoorn, D.M., Wu, Y.C., Xie, H.M., et al., 2009. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS ONE, 4(9):e7181. [doi:10.1371/journal.pone.0007181]
Eades, G., Yao, Y., Yang, M.H., et al., 2011. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J. Biol. Chem., 286(29):25992–26002. [doi:10.1074/jbc.M111.229401]
Ebert, M.S., Neilson, J.R., Sharp, P.A., 2007. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods, 4(9):721–726. [doi:10.1038/nmeth1079]
Esquela-Kerscher, A., Trang, P., Wiggins, J.F., et al., 2008. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle, 7(6):759–764. [doi:10.4161/cc.7.6.5834]
Farazi, T.A., Horlings, H.M., ten Hoeve, J.J., et al., 2011. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res., 71(13):4443–4453. [doi:10.1158/0008-5472.CAN-11-0608]
Frankel, L.B., Christoffersen, N.R., Jacobsen, A., et al., 2008. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem., 283(2):1026–1033. [doi:10.1074/jbc.M707224200]
Gao, J., Li, L.S., Wu, M.Q., et al., 2013. miR-26a inhibits proliferation and migration of breast cancer through repression of MCL-1. PLoS ONE, 8(6):e65138. [doi:10.1371/journal.pone.0065138]
Gilad, S., Meiri, E., Yogev, Y., et al., 2008. Serum microRNAs are promising novel biomarkers. PLoS ONE, 3(9):e3148. [doi:10.1371/journal.pone.0003148]
Goldberger, N., Walker, R.C., Kim, C.H., et al., 2013. Inherited variation in miR-290 expression suppresses breast cancer progression by targeting the metastasis susceptibility gene Arid4b. Cancer Res., 73(8):2671–2681. [doi:10.1158/0008-5472.CAN-12-3513]
Gregory, P.A., Bracken, C.P., Bert, A.G., et al., 2008. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle, 7(20):3112–3118. [doi:10.4161/cc.7.20.6851]
Gumireddy, K., Young, D.D., Xiong, X., et al., 2008. Small-molecule inhibitors of microRNA miR-21 function. Angew. Chem. Int. Ed., 47(39):7482–7484. [doi:10.1002/anie.200801555]
Guttilla, I.K., White, B.A., 2009. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J. Biol. Chem., 284(35):23204–23216. [doi:10.1074/jbc.M109.031427]
Hanke, M., Hoefig, K., Merz, H., et al., 2010. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol. Oncol.-Semin. Orig. Invest., 28(6): 655–661. [doi:10.1016/j.urolonc.2009.01.027]
Haque, I., Banerjee, S., Mehta, S., et al., 2011. Cysteine-rich 61-connective tissue growth factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1-induced signaling protein-2 (WISP-2) regulates microRNA-10b via hypoxia-inducible factor-1α-TWIST signaling networks in human breast cancer cells. J. Biol. Chem., 286(50):43475–43485. [doi:10.1074/jbc.M111.284158]
He, T., Qi, F.F., Jia, L., et al., 2014. MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J. Pathol., 232(5):499–508. [doi:10.1002/path.4324]
Heneghan, H.M., Miller, N., Kerin, M.J., 2010. Circulating miRNA signatures: promising prognostic tools for cancer. J. Clin. Oncol., 28(29):e573–e574. [doi:10.1200/JCO.2010.29.8901]
Hermeking, H., 2012. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat. Rev. Cancer, 12(9):613–626. [doi:10.1038/nrc3318]
Heyn, H., Engelmann, M., Schreek, S., et al., 2011. MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. Int. J. Cancer, 129(12):2797–2806. [doi:10.1002/ijc.25962]
Hu, J.J., Guo, H., Li, H.Y., et al., 2012. miR-145 regulates epithelial to mesenchymal transition of breast cancer cells by targeting OCT4. PLoS ONE, 7(9):e45965. [doi:10.1371/journal.pone.0045965]
Hu, X.W., Guo, J.Y., Zheng, L., et al., 2013. The heterochronic microRNA let-7 inhibits cell motility by regulating the genes in the actin cytoskeleton pathway in breast cancer. Mol. Cancer Res., 11(3):240–250. [doi:10.1158/1541-7786.MCR-12-0432]
Huang, Q.H., Gumireddy, K., Schrier, M., et al., 2008. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol., 10(2):202–210. [doi:10.1038/ncb1681]
Hwang, M.S., Yu, N., Stinson, S.Y., et al., 2013. miR-221/222 targets adiponectin receptor 1 to promote the epithelial-to-mesenchymal transition in breast cancer. PLoS ONE, 8(6):e66502. [doi:10.1371/journal.pone.0066502]
Iorio, M.V., Croce, C.M., 2009. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol., 27(34): 5848–5856. [doi:10.1200/JCO.2009.24.0317]
Iorio, M.V., Ferracin, M., Liu, C.G., et al., 2005. MicroRNA gene expression deregulation in human breast cancer. Cancer Res., 65(16):7065–7070. [doi:10.1158/0008-5472.CAN-05-1783]
Iorio, M.V., Visone, R., Di Leva, G., et al., 2007. MicroRNA signatures in human ovarian cancer. Cancer Res., 67(18): 8699–8707. [doi:10.1158/0008-5472.CAN-07-1936]
Iorio, M.V., Casalini, P., Piovan, C., et al., 2009. MicroRNA-205 regulates HER3 in human breast cancer. Cancer Res., 69(6):2195–2200. [doi:10.1158/0008-5472.CAN-08-2920]
Jansson, M.D., Lund, A.H., 2012. MicroRNA and cancer. Mol. Oncol., 6(6):590–610. [doi:10.1016/j.molonc.2012.09.006]
Jiang, S.A., Zhang, H.W., Lu, M.H., et al., 2010. MicroRNA-155 functions as an oncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res., 70(8):3119–3127. [doi:10.1158/0008-5472.CAN-09-4250]
Johnson, S.M., Lin, S.Y., Slack, F.J., 2003. The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev. Biol., 259(2):364–379. [doi:10.1016/S0012-1606(03)00202-1]
Karnoub, A.E., Dash, A.B., Vo, A.P., et al., 2007. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449(7162):557–563. [doi:10.1038/nature06188]
Kim, V.N., 2005. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol., 6(5):376–385. [doi:10.1038/nrm1644]
Kong, W., He, L.L., Coppola, M., et al., 2010. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J. Biol. Chem., 285(23):17869–17879. [doi:10.1074/jbc.M110.101055]
Korner, C., Keklikoglou, I., Bender, C., et al., 2013. MicroRNA-31 sensitizes human breast cells to apoptosis by direct targeting of protein kinase C ɛ (PKCɛ). J. Biol. Chem., 288(12):8750–8761. [doi:10.1074/jbc.M112.414128]
Korpal, M., Ell, B.J., Buffa, F.M., et al., 2011. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature Med., 17(9): 1101–1108. [doi:10.1038/nm.2401]
Kota, J., Chivukula, R.R., O’Donnell, K.A., et al., 2009. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell, 137(6): 1005–1017. [doi:10.1016/j.cell.2009.04.021]
Kreso, A., Dick, J.E., 2014. Evolution of the cancer stem cell model. Cell Stem Cell, 14(3):275–291. [doi:10.1016/j.stem.2014.02.006]
Krützfeldt, J., Rajewsky, N., Braich, R., et al., 2005. Silencing of microRNAs in vivo with ‘antagomirs’. Nature, 438(7068): 685–689. [doi:10.1038/nature04303]
Lagos-Quintana, M., Rauhut, R., Yalcin, A., et al., 2002. Identification of tissue-specific microRNAs from mouse. Curr. Biol., 12(9):735–739. [doi:10.1016/S0960-9822(02)00809-6]
Lee, R.C., Feinbaum, R.L., Ambros, V., 1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 75(5):843–854. [doi:10.1016/0092-8674(93)90529-Y]
Lei, R., Tang, J., Zhuang, X., et al., 2014. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene, 33(10):1287–1296. [doi:10.1038/onc.2013.65]
Li, J., Zhang, Y., Zhang, W., et al., 2013. Genetic heterogeneity of breast cancer metastasis may be related to miR-21 regulation of TIMP-3 in translation. Int. J. Surg. Oncol., 2013:875078. [doi:10.1155/2013/875078]
Li, L.Z., Zhang, C.Z., Liu, L.L., et al., 2014. miR-720 inhibits tumor invasion and migration in breast cancer by targeting TWIST1. Carcinogenesis, 35(2):469–478. [doi:10.1093/carcin/bgt330]
Li, Q.Y., Zhu, F.F., Chen, P.X., 2012. miR-7 and miR-218 epigenetically control tumor suppressor genes RASSF1A and Claudin-6 by targeting HoxB3 in breast cancer. Biochem. Biophys. Res. Commun., 424(1):28–33. [doi:10.1016/j.bbrc.2012.06.028]
Li, X., Roslan, S., Johnstone, C.N., et al., 2013. miR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways. Oncogene, 33(31): 4077–4088. [doi:10.1038/onc.2013.370]
Liao, D., Liu, Z., Wrasidlo, W., et al., 2011. Synthetic enzyme inhibitor: a novel targeting ligand for nanotherapeutic drug delivery inhibiting tumor growth without systemic toxicity. Nanomed.-Nanotechnol., 7(6):665–673. [doi:10.1016/j.nano.2011.03.001]
Lim, Y.Y., Wright, J.A., Attema, J.L., et al., 2013. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J. Cell Sci., 126(10):2256–2266. [doi:10.1242/jcs.122275]
Liu, X.X., Li, X.J., Zhang, B., et al., 2011. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett., 585(9): 1363–1367. [doi:10.1016/j.febslet.2011.04.018]
Liu, Y., Zhao, J., Zhang, P.Y., et al., 2012. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med. Sci. Monitor, 18(8):Br299–Br308. [doi:10.12659/MSM.883262]
Luo, Q.F., Li, X.Y., Gao, Y., et al., 2013. miRNA-497 regulates cell growth and invasion by targeting cyclin E1 in breast cancer. Cancer Cell Int., 13(1):95. [doi:10.1186/1475-2867-13-95]
Ma, L., Teruya-Feldstein, J., Weinberg, R.A., 2007. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature, 449(7163):682–688. [doi:10.1038/nature06174]
Ma, L., Young, J., Prabhala, H., et al., 2010a. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol., 12(3):247–256. [doi:10.1038/Ncb2024]
Ma, L., Reinhardt, F., Pan, E., et al., 2010b. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol., 28(4):341–347. [doi:10.1038/nbt.1618]
Mani, S.A., Guo, W., Liao, M.J., et al., 2008. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133(4):704–715. [doi:10.1016/j.cell.2008.03.027]
Meister, G., Landthaler, M., Dorsett, Y., et al., 2004. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA, 10(3):544–550. [doi:10.1261/rna.5235104]
Melo, S.A., Esteller, M., 2011. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett., 585(13): 2087–2099. [doi:10.1016/j.febslet.2010.08.009]
Meng, F.Y., Henson, R., Lang, M., et al., 2006. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology, 130(7):2113–2129. [doi:10.1053/j.gastro.2006.02.057]
Meng, F.Y., Henson, R., Wehbe-Janek, H., et al., 2007. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology, 133(2):647–658. [doi:10.1053/j.gastro.2007.05.022]
Mertens-Talcott, S.U., Chintharlapalli, S., Li, M.R., et al., 2007. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res., 67(22):11001–11011. [doi:10.1158/0008-5472.CAN-07-2416]
Mitchell, P.S., Parkin, R.K., Kroh, E.M., et al., 2008. Circulating microRNAs as stable blood-based markers for cancer detection. PNAS, 105(30):10513–10518. [doi:10.1073/pnas.0804549105]
Mitra, A., Rostas, J.W., Dyess, D.L., et al., 2012. Micro-RNA-632 downregulates DNAJB6 in breast cancer. Lab. Invest., 92(9):1310–1317. [doi:10.1038/labinvest.2012.87]
Moriarty, C.H., Pursell, B., Mercurio, A.M., 2010. miR-10b targets Tiam1 implications for Rac activation and carcinoma migration. J. Biol. Chem., 285(27):20541–20546. [doi:10.1074/jbc.M110.121012]
Mukhtar, R.A., Nseyo, O., Campbell, M.J., et al., 2011. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert. Rev. Mol. Diagn., 11(1):91–100. [doi:10.1586/erm.10.97]
Nassirpour, R., Mehta, P.P., Baxi, S.M., et al., 2013. miR-221 promotes tumorigenesis in human triple negative breast cancer cells. PLoS ONE, 8(4):e62170. [doi:10.1371/journal.pone.0062170]
Ng, E.K.O., Li, R.F.N., Shin, V.Y., et al., 2013. Circulating microRNAs as specific biomarkers for breast cancer detection. PLoS ONE, 8(1):e53141. [doi:10.1371/journal.pone.0053141]
Nguyen, D.P., Li, J., Yadav, S.S., et al., 2014. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int., 114(2):168–176. [doi:10.1111/bju.12488]
Nimmo, R.A., Slack, F.J., 2009. An elegant mirror: microRNAs in stem cells, developmental timing and cancer. Chromosoma, 118(4):405–418. [doi:10.1007/s00412-009-0210-z]
O’Hara, S.P., Mott, J.L., Splinter, P.L., et al., 2009. MicroRNAs: key modulators of posttranscriptional gene expression. Gastroenterology, 136(1):17–25. [doi:10.1053/j.gastro.2008.11.028]
Okuda, H., Xing, F., Pandey, P.R., et al., 2013. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res., 73(4):1434–1444. [doi:10.1158/0008-5472.CAN-12-2037]
Ørom, U.A., Kauppinen, S., Lund, A.H., 2006. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene, 372:137–141. [doi:10.1016/j.gene.2005.12.031]
Ovcharenko, D., Kelnar, K., Johnson, C., et al., 2007. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res., 67(22):10782–10788. [doi:10.1158/0008-5472.CAN-07-1484]
Park, N.J., Zhou, H., Elashoff, D., et al., 2009. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res., 15(17): 5473–5477. [doi:10.1158/1078-0432.CCR-09-0736]
Png, K.J., Yoshida, M., Zhang, X.H.F., et al., 2011. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev., 25(3):226–231. [doi:10.1101/gad.1974211]
Png, K.J., Halberg, N., Yoshida, M., et al., 2012. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature, 481(7380):190–194. [doi:10.1038/nature10661]
Qi, J., Wang, J., Katayama, H., et al., 2013. Circulating microRNAs (cmiRNAs) as novel potential biomarkers for hepatocellular carcinoma. Neoplasma, 60(2):135–142. [doi:10.4149/neo_2013_018]
Radojicic, J., Zaravinos, A., Vrekoussis, T., et al., 2011. MicroRNA expression analysis in triple-negative (ER, PR and Her2/neu) breast cancer. Cell Cycle, 10(3):507–517. [doi:10.4161/cc.10.3.14754]
Rivas, M.A., Venturutti, L., Huang, Y.W., et al., 2012. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res., 14(3): R77. [doi:10.1186/bcr3187]
Rubin, R., Arzumanyan, A., Soliera, A.R., et al., 2007. Insulin receptor substrate (IRS)-1 regulates murine embryonic stem (mES) cells self-renewal. J. Cell. Physiol., 213(2): 445–453. [doi:10.1002/jcp.21185]
Sachdeva, M., Mo, Y.Y., 2010. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res., 70(1):378–387. [doi:10.1158/0008-5472.CAN-09-2021]
Schee, K., Boye, K., Abrahamsen, T.W., et al., 2012. Clinical relevance of microRNA miR-21, miR-31, miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer. BMC Cancer, 12(1):505. [doi:10.1186/1471-2407-12-505]
Scheel, C., Eaton, E.N., Li, S.H.J., et al., 2011. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell, 145(6):926–940. [doi:10.1016/j.cell.2011.04.029]
Shipitsin, M., Polyak, K., 2008. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab. Invest., 88(5):459–463. [doi:10.1038/labinvest.2008.14]
Si, M.L., Zhu, S., Wu, H., et al., 2007. miR-21-mediated tumor growth. Oncogene, 26(19):2799–2803. [doi:10.1038/sj.onc.1210083]
Sica, A., Larghi, P., Mancino, A., et al., 2008. Macrophage polarization in tumour progression. Semin. Cancer Biol., 18(5):349–355. [doi:10.1016/j.semcancer.2008.03.004]
Smith, A.L., Iwanaga, R., Drasin, D.J., et al., 2012. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene, 31(50):5162–5171. [doi:10.1038/onc.2012.11]
Song, S.J., Poliseno, L., Song, M.S., et al., 2013. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell, 154(2):311–324. [doi:10.1016/j.cell.2013.06.026]
Sorlie, T., Perou, C.M., Tibshirani, R., et al., 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS, 98(19):10869–10874. [doi:10.1073/pnas.191367098]
Sossey-Alaoui, K., Downs-Kelly, E., Das, M., et al., 2011. WAVE3, an actin remodeling protein, is regulated by the metastasis suppressor microRNA, miR-31, during the invasion-metastasis cascade. Int. J. Cancer, 129(6): 1331–1343. [doi:10.1002/ijc.25793]
Spizzo, R., Nicoloso, M.S., Lupini, L., et al., 2010. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Differ., 17(2):246–254. [doi:10.1038/cdd.2009.117]
Stinson, S., Lackner, M.R., Adai, A.T., et al., 2011. miR-221/222 targeting of trichorhinophalangeal 1 (TRPS1) promotes epithelial-to-mesenchymal transition in breast cancer. Sci. Signal., 4(186):pt5. [doi:10.1126/scisignal.2002258]
Sun, X., Fan, C., Hu, L.J., et al., 2012. Role of let-7 in maintaining characteristics of breast cancer stem cells. Chin. J. Cell. Mol. Immunol., 28(8):789–792 (in Chinese).
Tang, W., Yu, F., Yao, H., et al., 2014. miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene, 33(20):2629–2638. [doi:10.1038/onc.2013.214]
Tanic, M., Yanowsky, K., Rodriguez-Antona, C., et al., 2012. Deregulated miRNAs in hereditary breast cancer revealed a role for miR-30c in regulating KRAS oncogene. PLoS ONE, 7(6):e38847. [doi:10.1371/journal.pone.0038847]
Tanzer, A., Stadler, P.F., 2004. Molecular evolution of a microRNA cluster. J. Mol. Biol., 339(2):327–335. [doi:10.1016/j.jmb.2004.03.065]
Tavazoie, S.F., Alarcon, C., Oskarsson, T., et al., 2008. Endogenous human microRNAs that suppress breast cancer metastasis. Nature, 451(7175):147–152. [doi:10.1038/nature06487]
Taylor, M.A., Sossey-Alaoui, K., Thompson, C.L., et al., 2013. TGF-β upregulates miR-181a expression to promote breast cancer metastasis. J. Clin. Invest., 123(1):150–163. [doi:10.1172/JCI64946]
Tsuchiya, Y., Nakajima, M., Takagi, S., et al., 2006. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res., 66(18):9090–9098. [doi:10.1158/0008-5472.CAN-06-1403]
Valastyan, S., Chang, A., Benaich, N., et al., 2011. Activation of miR-31 function in already-established metastases elicits metastatic regression. Genes Dev., 25(6):646–659. [doi:10.1101/gad.2004211]
Valent, P., Bonnet, D., de Maria, R., et al., 2012. Cancer stem cell definitions and terminology: the devil is in the details. Nat. Rev. Cancer, 12(11):767–775. [doi:10.1038/nrc3368]
Wang, B., Zhang, Q.Y., 2012. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J. Cancer Res. Clin. Oncol., 138(10):1659–1666. [doi:10.1007/s00432-012-1244-9]
Wang, F.J., Zheng, Z.G., Guo, J.F., et al., 2010. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol. Oncol., 119(3):586–593. [doi:10.1016/j.ygyno.2010.07.021]
Wang, S., Huang, J., Lyu, H., et al., 2013. Functional cooperation of miR-125a, miR-125b, and miR-205 in entinostat-induced downregulation of erbB2/erbB3 and apoptosis in breast cancer cells. Cell Death Dis., 4(3): e556. [doi:10.1038/cddis.2013.79]
Wang, S.H., Bian, C.J., Yang, Z., et al., 2009. miR-145 inhibits breast cancer cell growth through RTKN. Int. J. Oncol., 34(5):1461–1466. [doi:10.3892/Ijo_00000275]
Weidhaas, J.B., Babar, L., Nallur, S.M., et al., 2007. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res., 67(23):11111–11116. [doi:10.1158/0008-5472.CAN-07-2858]
Wu, H.L., Zhu, S.M., Mo, Y.Y., 2009. Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res., 19(4):439–448. [doi:10.1038/cr.2009.18]
Xu, Q., Jiang, Y., Yin, Y., et al., 2013. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J. Mol. Cell Biol., 5(1):3–13. [doi:10.1093/Jmcb/Mjs049]
Yanaihara, N., Caplen, N., Bowman, E., et al., 2006. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell, 9(3):189–198. [doi:10.1016/j.ccr.2006.01.025]
Yang, J., Zhang, Z., Chen, C., et al., 2014. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through down-regulated expression of Fra-1 proto-oncogene. Oncogene, 33(23): 3014–3023. [doi:10.1038/onc.2013.258]
Yu, F., Yao, H., Zhu, P.C., et al., 2007. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell, 131(6):1109–1123. [doi:10.1016/j.cell.2007.10.054]
Yu, F., Deng, H., Yao, H., et al., 2010. miR-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene, 29(29):4194–4204. [doi:10.1038/onc.2010.167]
Yu, Z.R., Wang, C.G., Wang, M., et al., 2008. A Cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol., 182(3): 509–517. [doi:10.1083/jcb.200801079]
Zhang, C.M., Zhao, J., Deng, H.Y., 2013. miR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein 1. J. Biomed. Sci., 20(1):79. [doi:10.1186/1423-0127-20-79]
Zhou, J., Tian, Y., Li, J., et al., 2013. miR-206 is down-regulated in breast cancer and inhibits cell proliferation through the up-regulation of cyclinD2. Biochem. Biophys. Res. Commun., 433(2):207–212. [doi:10.1016/j.bbrc.2013.02.084]
Zhu, S., Sachdeva, M., Wu, F., et al., 2010. Ubc9 promotes breast cell invasion and metastasis in a sumoylation-independent manner. Oncogene, 29(12):1763–1772. [doi:10.1038/onc.2009.459]
Zhu, S.M., Si, M.L., Wu, H.L., et al., 2007. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J. Biol. Chem., 282(19):14328–14336. [doi:10.1074/jbc.M611393200]
Zoon, C.K., Starker, E.Q., Wilson, A.M., et al., 2009. Current molecular diagnostics of breast cancer and the potential incorporation of microRNA. Expert Rev. Mol. Diagn., 9(5):455–467. [doi:10.1586/erm.09.25]
Zou, C., Xu, Q., Mao, F., et al., 2012. miR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle, 11(11):2137–2145. [doi:10.4161/cc.20598]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Basic Research Program (973) of China (No. 2013CB967202) and the National Natural Science Foundation of China (No. 81472654)
ORCID: Yun-ping LUO, http://orcid.org/0000-0001-6905-0768/0000-0001-6905-0768
Rights and permissions
About this article
Cite this article
Wang, W., Luo, Yp. MicroRNAs in breast cancer: oncogene and tumor suppressors with clinical potential. J. Zhejiang Univ. Sci. B 16, 18–31 (2015). https://doi.org/10.1631/jzus.B1400184
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1400184