Abstract
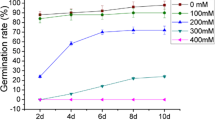
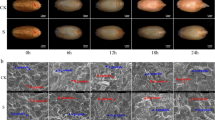
Soybean (Glycine max (L.) Merrill) is a salt-sensitive crop, and its production is severely affected by saline soils. Therefore, the response of soybean seeds to salt stress during germination was investigated at both physiological and proteomic levels. The salt-tolerant cultivar Lee68 and salt-sensitive cultivar N2899 were exposed to 100 mmol/L NaCl until radicle protrusion from the seed coat. In both cultivars, the final germination percentage was not affected by salt, but the mean germination times of Lee68 and N2899 were delayed by 0.3 and 1.0 d, respectively, compared with controls. In response to salt stress, the abscisic acid content increased, and gibberellic acid (GA1+3) and isopentenyladenosine decreased. Indole-3-acetic acid increased in Lee68, but remained unchanged in N2899. The proteins extracted from germinated seeds were separated using two-dimensional gel electrophoresis (2-DE), followed by Coomassie brilliant blue G-250 staining. About 350 protein spots from 2-DE gels of pH range 3 to 10 and 650 spots from gels of pH range 4 to 7 were reproducibly resolved, of which 18 protein spots showed changes in abundance as a result of salt stress in both cultivars. After matrix-assisted laser desorption ionization-time of flight-mass spectroscopy (MALDI-TOF-MS) analysis of the differentially expressed proteins, the peptide mass fingerprint was searched against the soybean UniGene database and nine proteins were successfully identified. Ferritin and 20S proteasome subunit β-6 were up-regulated in both cultivars. Glyceraldehyde 3-phosphate dehydrogenase, glutathione S-transferase (GST) 9, GST 10, and seed maturation protein PM36 were down-regulated in Lee68 by salt, but still remained at a certain level. However, these proteins were present in lower levels in control N2899 and were up-regulated under salt stress. The results indicate that these proteins might have important roles in defense mechanisms against salt stress during soybean seed germination.
Similar content being viewed by others
References
Aghaei, K., Ehsanpour, A.A., Shah, A.H., Komatsu, S., 2008. Proteome analysis of soybean hypocotyl and root under salt stress. Amino Acids, 36(1):91–98. [doi:10.1007/s00726-008-0036-7]
Agrawal, G.K., Hajduch, M., Graham, K., Thelen, J.J., 2008. In-depth investigation of soybean seed-filling proteome and comparison with a parallel study of rapeseed. Plant Physiol., 148(1):504–518. [doi:10.1104/pp.108.119222]
Alam, I., Sharmin, S.A., Kim, K.H., Yang, J.K., Choi, M.S., Lee, B.H., 2010. Proteome analysis of soybean roots subjected to short-term drought stress. Plant Soil, 333(1–2):491–505. [doi:10.1007/s11104-010-0365-7]
Almansouri, M., Kinet, J.M., Lutts, S., 2001. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil, 231(2):243–254. [doi:10.1023/A:1010378409663]
Bianco, C., Defez, R., 2009. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot., 60(11):3097–3107. [doi:10.1093/jxb/erp140]
Blackman, S.A., Wettlaufer, S.H., Obendorf, R.L., Leopold, A.C., 1991. Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol., 96(3):868–874. [doi:10.1104/pp.96.3.868]
Boyer, J.S., 1982. Plant productivity and environment. Science, 218(4571):443–448. [doi:10.1126/science.218.4571.443]
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem., 72(1–2):248–254. [doi:10.1016/0003-2697(76)90527-3]
Briat, J.F., Lobréaux, S., Grignon, N., 1999. Regulation of plant ferritin synthesis: how and why. Cell. Mol. Life Sci., 56(1–2):155–166. [doi:10.1007/s000180050014]
Chen, J.G., Du, X.M., Zhou, X., Zhao, H.Y., 1997. Levels of cytokinins in the ovules of cotton mutants with altered fiber development. J. Plant Growth Regul., 16(3): 181–185. [doi:10.1007/PL00006994]
Chen, J.G., Zhou, X., Zhang, Y.Z., 1998a. Gibberellin-responding and non-responding dwarf mutants in foxtail millet. Plant Growth Regul., 26(1):19–24. [doi:10.1023/A:1006091601256]
Chen, J.G., Cheng, S.H., Cao, W.X., Zhou, X., 1998b. Involvement of endogenous plant hormones in the effect of mixed nitrogen source on growth and tillering of wheat. J. Plant Nutr., 21(1):87–97. [doi:10.1080/01904169809365385]
Cheng, L., Gao, X., Li, S., Shi, M., Javeed, H., Jing, X., Yang, G., He, G., 2010. Proteomic analysis of soybean [Glycine max (L.) Meer.] seeds during imbibition at chilling temperature. Mol. Breeding, 26(1):1–17. [doi:10.1007/s11032-009-9371-y]
Duée, E., Olivier-Deyris, L., Fanchon, E., Corbier, C., Branlant, G., Dideberg, O., 1996. Comparison of the structures of wild-type and a N313T mutant of Escherichia coli glyceraldehyde 3-phosphate dehydrogenases: implication for NAD binding and cooperativity. J. Mol. Biol., 257(4):814–838. [doi:10.1006/jmbi.1996.0204]
Gallardo, K., Job, C., Groot, S.P., Puype, M., Demol, H., Vandekerckhove, J., Job, D., 2002. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol., 129(2):823–837. [doi:10.1104/pp.002816]
Gidrol, X., Lin, W.S., Dégousée, N., Yip, S.F., Kush, A., 1994. Accumulation of reactive oxygen species and oxidation of cytokinin in germinating soybean seeds. Eur. J. Biochem., 224(1):21–28. [doi:10.1111/j.1432-1033.1994.tb19990.x]
Gygi, S.P., Aebersold, R., 2000. Mass spectrometry and proteomics. Curr. Opin. Chem. Biol., 4(5):489–494. [doi:10.1016/S1367-5931(00)00121-6]
Hajduch, M., Ganapathy, A., Stein, J.W., 2005. A systematic proteomic study of seed filling in soybean. Establishment of high-resolution two-dimensional reference maps, expression profiles, and an interactive proteome database. Plant Physiol., 137(4):1397–1419. [doi:10.1104/pp.104.056614]
Hamayun, M., Khan, S.A., Shinwari, Z.K., Khan, A.L., Ahmad, N., Lee, I.J., 2010a. Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Pak. J. Bot., 42(5): 3103–3112.
Hamayun, M., Khan, S.A., Khan, A.L., Shin, J.H., Ahmad, B., Shin, D.H., Lee, I.J., 2010b. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem., 58(12):7226–7232. [doi: 10.1021/jf101221t]
Hsing, Y.C., Tsou, C.H., Hsu, T.F., Chen, Z.Y., Hsieh, K.L., Hsieh, J.S., Chow, T.Y., 1998. Tissue- and stage-specific expression of a soybean (Glycine max L.) seed-maturation biotinylated protein. Plant Mol. Biol., 38(3): 481–490. [doi:10.1023/A:1006079926339]
Imin, N., Kerim, T., Weinman, J.J., Rolfe, B.G., 2006. Low temperature treatment at the young microspore stage induces protein changes in rice anthers. Mol. Cell. Proteom., 5(2):274–292. [doi:10.1074/mcp.M500242-MCP200]
Itoh, H., Matsuoka, M., Camille, M., 2003. A role for the ubiquitin-26S proteasome pathway in gibberellin signaling. Trends Plant Sci., 8(10):492–497. [doi:10.1016/j.tplants.2003.08.002]
Jeong, M.J., Park, S.C., Byun, M.O., 2001. Improvement of salt tolerance in transgenic potato plants by glyceraldehyde-3-phosphate dehydrogenase gene transfer. Mol. Cell, 12(2):185–189.
Jia, G.X., Zhu, Z.Q., Chang, F.Q., Li, Y.X., 2002. Transformation of tomato with the BADH gene from Atriplex improves salt tolerance. Plant Cell Rep., 21(2):141–146. [doi:10.1007/s00299-002-0489-1]
Kaur, S., Gupta, A.K., Kaur, N., 1998. Gibberellin A3 reverses the effect of salt stress in chickpea (Cicerarietinum L.) seedlings by enhancing amylase activity and mobilization of starch in cotyledons. Plant Growth Regul., 26(2): 85–90. [doi:10.1023/A:1006008711079]
Kim, S.T., Kang, S.Y., Wang, Y., Kim, S.G., Hwang, D.H., Kang, K.Y., 2008. Analysis of embryonic proteome modulation by GA and ABA from germinating rice seeds. Proteomics, 8(17):3577–3587. [doi:10.1002/pmic.200800183]
Levitt, J., 1980. Responses of Plants to Environmental Stresses: Water, Radiation, Salt and Other Stresses. Academic Press, New York, Vol. 2, p.365–402.
Lobréaux, S., Hardy, T., Briat, J.F., 1993. Abscisic acid is involved in the iron-induced synthesis of maize ferritin. EMBO J., 12(2):651–657.
Luo, Q.Y., Yu, B.J., Liu, Y.L., 2005. Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J. Plant Physiol., 162(9): 1003–1012. [doi:10.1016/j.jplph.2004.11.008]
Marrs, K.A., 1996. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 47(1):127–158. [doi:10.1146/annurev.arplant.47.1.127]
Masuda, T., Goto, F., Yoshihara, T., 2001. A novel plant ferritin subunit from soybean that is related to a mechanism in iron release. J. Biol. Chem., 276(22):19575–19579. [doi:10.1074/jbc.M011399200]
McGonigle, B., Keeler, S.J., Lau, S.M.C., 2000. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol., 124(3):1105–1120. [doi:10.1104/pp.124.3.1105]
Mooney, B.P., Thelen, J.J., 2004. High-throughput peptide mass fingerprinting of soybean seed proteins: automated workflow and utility of UniGene expressed sequence tag databases for protein identification. Phytochemistry, 65(3):1733–1744. [doi:10.1016/j.phytochem.2004.04.011]
Morgan, P.W., 1990. Effects of Abiotic Stresses on Plant Hormone Systems. In: Alscher, R.G., Cumming, J.R. (Eds.), Stress Responses in Plants: Adaptation and Acclimation Mechanism. Wiley-Liss, New York.
Munns, R., 2005. Genes and salt tolerance: bringing them together. New Phytol., 167(3):645–660. [doi:10.1111/j.1469-8137.2005.01487.x]
Neuhoff, V., Arold, N., Taube, D., Ehrhardt, W., 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis, 9(6):205–262. [doi: 10.1002/elps.1150090603]
Nouri, M.Z., Komatsu, S., 2010. Comparative analysis of soybean plasma membrane proteins under osmotic stress using gel-based and LC MS/MS-based proteomics approaches. Proteomics, 10(10):1930–1945. [doi:10.1002/pmic.200900632]
Nouri, M.Z., Toorchi, M., Komatsu, S., 2011. Proteomics Approach for Identifying Abiotic Stress Responsive Proteins in Soybean. In: Sudaric, A. (Ed.), Soybean-Molecular Aspects of Breeding. InTech, Croatia, p.187–214.
Ravet, K., Touraine, B., Boucherez, J., Briat, J.F., Gaymard, F., Cellier, F., 2009. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J., 57(3):400–412. [doi:10.1111/j.1365-313X.2008.03698.x]
Roxas, V.P., Smith, R.K., Allen, E.R., 1997. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat. Biotechnol., 15(10):988–991. [doi:10. 1038/nbt1097-988]
Sassa, H., Oguchi, S., Inoue, T., 2000. Primary structural features of the 20S proteasome subunits of rice (Oryza sativa). Gene, 250(1–2):61–66. [doi:10.1016/S0378-1119 (00)00190-6]
Skriver, K., Mundy, J., 1990. Gene expression in response to abscisic acid and osmotic stress. Plant Cell, 2(6):503–512. [doi:10.1105/tpc.2.6.503]
Smalle, J., Vierstra, R.D., 2004. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol., 55(1): 555–590. [doi:10.1146/annurev.arplant.55.031903.141801]
Smith, S.M., 2002. Does the glyoxylate cycle have an anaplerotic function in plants? Trends Plant Sci., 7(1): 12–13. [doi:10.1016/S1360-1385(01)02189-6]
Sobhanian, H., Razavizadeh, R., Nanjo, Y., Ehsanpour, A.A., RastgarJazii, F., Motamed, N., Komatsu, S., 2010. Proteome analysis of soybean leaves, hypocotyls and roots under salt stress. Proteome Sci., 8(1):19 [doi:10.1186/1477-5956-8-19]
Toorchi, M., Yukawa, K., Nouri, M.Z., Komatsu, S., 2009. Proteomics approach for identifying osmotic-stress-related proteins in soybean roots. Peptides, 30(12): 2108–2117. [doi:10.1016/j.peptides.2009.09.006]
Umezawa, T., Shimizu, K., Kato, M., Ueda, T., 2001. Effects of non-stomatal components on photosynthesis in soybean under salt stress. Jpn. J. Trop. Agric., 45(1):57–63.
Watson, B.S., Asirvatham, V.S., Wang, L.J., Sumner, L.W., 2003. Mapping the proteome of Barrel Medic (Medicagotruncatula). Plant Physiol., 131(3):1104–1123. [doi:10.1104/pp.102.019034]
Wei, A.L., Chen, Y.Z., 2000. Effect of IAA on soybean seedling’s membrance injury and salt resistance. Acta Bot. Boreal. Occident. Sin., 20(3):410–414 (in Chinese).
Xu, X.Y., Zheng, R., Li, C.M., Gai, J.Y., Yu, D.Y., 2006. Differential proteomic analysis of seed germination in soybean. Prog. Biochem. Biophys., 33(11):1106–1112 (in Chinese).
Ying, L.U., Wu, Y.R., Han, B., 2005. Anaerobic induction of isocitrate lyase and malate synthase in submerged rice seedlings indicates the important metabolic role of the glyoxylate cycle. Acta Biochem. Biophys. Sin., 37(6): 406–414.
Zhen, Y., Qi, J.L., Wang, S.S., Su, J., Xu, G.H., Zhang, M.S., Miao, L., Peng, X.X., Tian, D., Yang, Y.H., 2007. Comparative proteome analysis of differentially expressed proteins induced by Al toxicity in soybean. Physiol. Plant., 131(4):542–554. [doi:10.1111/j.1399-3054.2007.00979.x]
Zhu, J.K., 2002. Salt and drought stress signals transduction in plants. Annu. Rev. Plant Biol., 53(1):247–273. [doi:10.1146/annurev.arplant.53.091401.143329]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No. 30800692), the National Basic Research Program (973) of China (Nos. 2010CB125906 and 2009CB118400), and the National High-Tech R & D Program (863) of China (No. 2006AA10Z1C1)
Rights and permissions
About this article
Cite this article
Xu, Xy., Fan, R., Zheng, R. et al. Proteomic analysis of seed germination under salt stress in soybeans. J. Zhejiang Univ. Sci. B 12, 507–517 (2011). https://doi.org/10.1631/jzus.B1100061
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1100061