Abstract
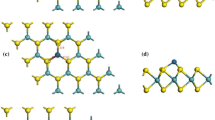
Elemental mercury has become a global concern because of its significant impact on human health and the ecosystem. A lot of effort has been put towards the removal of elemental mercury from the 2H-MoS2 (prismatic structure of MoS2). However, the mechanism of 1T-MoS2 (polytype structure of MoS2) in Hg0 capture remains unexplored. In this study, density functional theory (DFT) was adopted to investigate the adsorption mechanism of Hg on a 1T-MoS2 monolayer. The different possible adsorption positions on the 1T-MoS2 were examined. For different adsorption configurations, the changes in electronic property were also studied to understand the adsorption process. The results elucidated that chemisorption dominates the adsorption between Hg0 atoms and the 1T-MoS2. It was found that the TMo (on top of the Mo atom) position is the strongest adsorption configuration among all the possible adsorption positions. The adsorption of Hg0 atoms on the 1T-MoS2 monolayer is influenced by adjacent S and Mo atoms. The adsorbate Hg0 atom is found being oxidized on the TMo position of the 1T-MoS2 with an adsorption energy of −1.091 eV. From the partial density of states (PDOS) analysis of the atoms, the strong interaction between Hg0 and the 1T-MoS2 surface is caused by the significant overlap among the d orbitals of the mercury atom and the s orbital of the S atom and p and d orbitals of the Mo atom.
摘要
目 的
探索1T-MoS2 (多型结构的二硫化钼) 的除汞机制。
方 法
1. 采用密度泛函理论 (DFT) 分析Hg0 在1T-MoS2单层上的吸附机理。2. 考察1T-MoS2的不同吸附位置。3. 对不同的吸附构型, 研究电子吸附前后的变化, 从而进一步了解吸附过程。
结 论
1. 化学吸附是Hg 原子与1T-MoS2单层吸附的主导因素。同时, 在所有可能的吸附位置中, TMo (在钼原子上方) 的位置是最强烈的吸附构型。 2. 汞 (Hg) 原子在1T-MoS2单层上的吸附受邻近 的硫 (S) 和钼 (Mo) 原子的影响。3. 吸附的汞 (Hg) 原子在1T-MoS2的TMo位置上会被氧化, 其吸附能为−1.091 eV。4. 从局部态密度 (PDOS) 分析来看, Hg 原子和1T-MoS2表面之间的相互作 用是由汞 (Hg) 原子的d 轨道与硫 (S) 原子的s 轨道及钼 (Mo)原子的p 轨道和d 轨道重叠所致。
Similar content being viewed by others
References
Aboud S, Sasmaz E, Wilcox J, 2008. Mercury adsorption on PdAu, PdAg and PdCu alloys. Main Group Chemistry, 7(3):205–215. https://doi.org/10.1080/10241220802465213
Asasian N, Kaghazchi T, 2015. Sulfurized activated carbons and their mercury adsorption/desorption behavior in aqueous phase. International Journal of Environmental Science and Technology, 12(8):2511–2522. https://doi.org/10.1007/s13762-015-0818-x
Atkins PW, 2001. Physical Chemistry. Oxford University Press, UK.
Chadi DJ, 1977. Special points for Brillouin-zone integratuibs. Physical Review B, 13:5188–5192.
Chhowalla M, Shin HS, Eda G, et al., 2013. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nature Chemistry, 5(4):263–275. https://doi.org/10.1038/nchem.1589
Clark SJ, Segall MD, Pickard CJ, et al., 2005. First principles methods using CASTEP. Zeitschrift Fur Kristallographie, 220:567–570.
Delley B, 1986. Calculated electron distribution for terafluoroterephthalonitrile (TFT). Chemical Physics, 110(2-3):329–338. https://doi.org/10.1016/0301-0104(86)87089-6
EPA (Environmental Protection Agency), 2011. National Emission Standards for Hazardous Air Pollutants from Coil-and Oil-fired Electric Utility Steam Generating Units and Standards of Performance for Fossil-Fuel-Fired Electric Utility, Industrial-Commercial-Institutional, and Small Industrial-Commercial-Institutional Steam Generating Units. https://www.federalregister.gov/articles/2016/04/ 06/2016-06563/national-emission-standards-for-hazardo us-air-pollutants-from-coal—and-oil-fired-electric-utility [Accessed on Feb. 15, 2016].
Enyashin AN, Seifert G, 2012. Density-functional study of LixMoS2 intercalates (0≤x≤1). Computational and Theoretical Chemistry, 999:13–20. https://doi.org/10.1016/j.comptc.2012.08.005
Galbreath KC, Zygarlicke CJ, 1996. Mercury speciation in coal combustion and gasification flue gases. Environmental Science & Technology, 30(8):2421–2426. https://doi.org/10.1021/es950935t
Gao G, Jiao Y, Ma F, et al., 2015. Charge mediated semiconducting-to-metallic phase transition in molybdenum disulfide monolayer and hydrogen evolution reaction in new 1T’ phase. The Journal of Physical Chemistry C, 119(23):13124–13128. https://doi.org/10.1021/acs.jpcc.5b04658
Gao Y, Zhang Z, Wu J, et al., 2013. A critical review on the heterogeneous catalytic oxidation of elemental mercury in flue gases. Environmental Science & Technology, 47(19):10813–10823. https://doi.org/10.1021/es402495h
Eda G, Fujita T, Yamaguchi H, et al., 2012. Coherent atomic and electronic heterostructures of single-layer MoS2. ACS Nano, 6(8):7311–7317. https://doi.org/10.1021/nn302422x
Hasnip PJ, Refson K, Probert MI, et al., 2014. Density functional theory in the solid state. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 372:20130270. https://doi.org/10.1098/rsta.2013.0270
Huang Z, Hao G, He C, et al., 2013. Density functional theory study of Fe adatoms adsorbed monolayer and bilayer MoS2 sheets. Journal of Applied Physics, 114:083706. https://doi.org/10.1063/1.4818952
Johansen VC, 2003. Mercury speciation in other combustion sources: a literature review. Portland Cement Association, 2578:20.
Jones DW, 1999. Exposure or absorption and the crucial question of limits for mercury. Journal of the Canadian Dental Association, 65(1):42–46.
Liang S, Wang Y, Cinnirella S, et al., 2015. Atmospheric mercury footprints of nations. Environmental Science & Technology, 49(6):3566–3574. https://doi.org/10.1021/es503977y
Lim D, Wilcox J, 2013. Heterogeneous mercury oxidation on Au(111) from first principles. Environmental Science & Technology, 47(15):8515–8522. https://doi.org/10.1021/es400876e
Liu D, Chen X, Li D, 2010. Simulation of MoS2 crystal structure and the experimental study of thermal decomposition. Journal of Molecular Structure, 980(1-3):66–71. https://doi.org/10.1016/j.molstruc.2010.06.038
Ortmann F, Bechstedt F, Schmidt WG, 2006. Semiempirical van der Waals correction to the density functional description of solids and molecular structures. Physical Review B, 73(20):205101. https://doi.org/10.1103/PhysRevB.73.205101
Pfrommer BG, Cote M, Louie SG, et al., 1997. Relaxation of crystals with the quasi-Newton method. Journal of Computational Physics, 131:233–240. https://doi.org/10.1006/jcph.1996.5612
Praveen A, 2003. Mercury Emissions from Coal Fired Power Plants. Northeast States for Coordinated Air Use Management.
Presto AA, Granite EJ, 2006. Survey of Catalysts for Oxidation of Mercury in Flue Gas. Environmental Science & Technology, 40(18):5601–5609. https://doi.org/10.1021/es060504i
Putungan DB, Kuo JL, 2014. Structural and electronic properties of monolayer 1T-MoS2 phase, and its interaction with water adsorbed on perfect, single S-vacated and MoS2-unit-vacated surface: density functional theory calculations. Integrated Ferroelectrics, 156(1):93–101. https://doi.org/10.1080/10584587.2014.906790
Sato T, Nakai H, 2009. Density functional method including weak interactions: dispersion coefficients based on the local response approximation. The Journal of Chemical Physics, 131(22):224104. https://doi.org/10.1063/1.3269802
UNEP (United Nations Environment Programme), 2002. Global Mercury Assessment. http://www.unep.org [Accessed on Jan. 15, 2016].
UNEP (United Nations Environment Programme), 2013a. Final Act of the Conference of Plenipotentiaries on the Minamata Convention on Mercury. http://www. mercuryconvention.org [Accessed on Apr. 15, 2016].
UNEP (United Nations Environment Programme), 2013b. Global Mercury Assessment 2013: Sources, Emissions, Releases, and Environmental Transport. https://www. unep.org [Accessed on Apr. 15, 2016].
UNEP (United Nations Environment Programme), 2013c. The Minamata Convention on Mercury. https://www. mercuryconvention.org/Convention/tabid/3426/ [Accessed on Apr. 15, 2016].
Vanderbilt D, 1990. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Physical Review B, 41(11):7892–7895. https://doi.org/10.1103/PhysRevB.41.7892
Wypych F, Schollhorn R, 1992. 1T-MoS2, a new metallic modification of molybdenum disulfide. Journal of the Chemical Society, Chemical Communications, (19):1386–1388. https://doi.org/10.1039/C39920001386
Wypych F, Weber T, Prins R, 1998. Scanning tunneling microscopic investigation of 1T-MoS2. Chemistry of Materials, 10(3):723–727. https://doi.org/10.1021/cm970402e
Yin Z, Li H, Li H, et al., 2011. Single-layer MoS2 phototransistors. ACS Nano, 6(1):74–80. https://doi.org/10.1021/nn2024557
Zhao H, Yang G, Gao X, et al., 2016. Hg0 capture over Co-MoS/γ-Al2O3 with MoS2 nanosheets at low temperatures. Environmental Science & Technology, 50(2):1056–1064. https://doi.org/10.1021/acs.est.5b04278
Acknowledgements
The University of Nottingham Ningbo China is acknowledged for the provision of a full scholarship to the first author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Ningbo Bureau of Science and Technology (No. 2012B82011), the Ningbo Natural Science Foundation (No. 2017A610060), the National Natural Science Foundation of China (No. 51706114), and the China Postdoctoral Science Foundation (No. 2016M601942)
Rights and permissions
About this article
Cite this article
Mu, Xl., Gao, X., Zhao, Ht. et al. Density functional theory study of the adsorption of elemental mercury on a 1T-MoS2 monolayer. J. Zhejiang Univ. Sci. A 19, 60–67 (2018). https://doi.org/10.1631/jzus.A1700079
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A1700079