Abstract
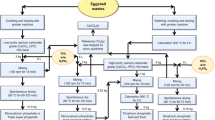
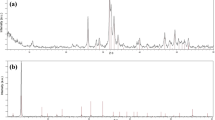
Eggshells wastes are produced in huge amounts worldwide. The recycling or valorization of this waste, which otherwise is usually disposed in landfills, represents an opportunity within a circular economy perspective. In the present work, the potential of chicken eggshell waste to produce calcitic lime was explored. After collection from an industry supplier, the waste was thoroughly characterized for its mineralogical, chemical, and thermal properties. The material was calcined at 1000 °C, and the obtained calcium oxide was evaluated for its reactivity in wet slaking tests. Comparison was made with commercial limestone used as reference. It was found that the calcium oxide from eggshell waste belonged to the most reactive class (R5—60 °C within 10 min), the same of the calcium oxide from limestone. However, different times were obtained to reach 60 °C (25 s and 4:37 min:s) and for 80% of the reaction (28 s and 5 min) for calcium oxide from limestone and eggshell waste, respectively. The lower reactivity of calcium oxide from eggshell waste was related to its larger size particles with smoother surfaces and lower specific surface area in comparison to limestone calcium oxide. Industrial, environmental and economic implications concerning the use of this waste to produce lime were also evaluated. The eggshell waste could be all consumed at an industrial scale in Portugal allowing for approximately 2.6% partial substitution of limestone in a lime factory.





Similar content being viewed by others
References
Rivera EM, Araiza M, Brostow W, Castaño VM, Díaz-Estrada JR, Hernández R, Rodríguez JR (1999) Synthesis of hydroxyapatite from eggshells. Mater Lett 41:128–134
Park S, Choi KS, Lee D, Kim D, Lim KT, Lee K-H, Seonwoo H, Kim J (2016) Eggshell membrane: review and impact on engineering. Biosyst Eng 151:446–463
Quina MJ, Soares MAR, Quinta-Ferreira R (2017) Applications of industrial eggshell as a valuable anthropogenic resource. Resour Conserv Recycl 123:176–186
Oliveira DA, Benelli P, Amante ER (2013) A literature review on adding value to solid residues: egg shells. J Clean Prod 46:42–47
Pliya P, Cree D (2015) Limestone derived eggshell powder as a replacement in Portland cement mortar. Constr Build Mater 95:1–9
Baláž M (2014) Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater 10:3827–3843
Viriya-Empikul N, Krasae P, Puttasawat B, Yoosuk B, Chollacoop N, Faungnawakij K (2010) Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour Technol 101:3765–3767
Mosaddegh E, Hassankhani A (2014) Preparation and characterization of nano-CaO based on eggshell waste: novel and green catalytic approach to highly efficient synthesis of pyrano[4,3-b]pyrans. Chin J Catal 35:351–356
Sacia ER, Ramkumar S, Phalak N, Fan L-S (2013) Synthesis and regeneration of sustainable CaO sorbents from chicken eggshells for enhanced carbon dioxide capture. ACS Sustain Chem Eng 1:903–909
Beck K, Brunetaud X, Mertz J.-D, Al-Mukhtar M (2010) On the use of eggshell lime and tuffeau powder to formulate an appropriate mortar for restoration purposes. In: Smith, BJ, Gomez-Heras M, Viles HA, Cassar J (eds) Limestone in the built environment: present-day challenges for the preservation of the past. Geological Society, London, Special Publications 331, pp 137–145
Jirimali HD, Chaudhari BC, Khanderay JC, Joshi SA, Singh V, Patil AM, Gite VV (2018) Waste eggshell-derived calcium oxide and nanohydroxyapatite biomaterials for the preparation of LLDPE polymer nanocomposite and their thermomechanical study. Polym-Plast Technol Eng 57:804–811
Tan YH, Abdullah MO, Nolasco-Hipolito C, Zauzi NSA (2017) Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew Energy 114(Part B):437–447
Nagabhushana KR, Lokesha HS, Reddy SS, Prakash D, Veerabhadraswamy M, Bhagyalakshmi H, Jayaramaiah JR (2017) Thermoluminescence properties of CaO powder obtained from chicken eggshells. Radiat Phys Chem 138:54–59
Frank G (1974) Determining the reactivity of limes by means of the wet slaking curve. ZKG Int 4:172–176
Potgieter JH, Potgieter SS, Moja SJ, Mulaba-Bafubiandi A (2002) The standard reactivity test as a measure of lime’s quality. J S Afr Inst Min Metall 102:67–69
Hogewoning S, Mehling C, Wettrau D, Wolter A, Bohne T (2011) Extension of the wet slaking curve evaluation to include determination of the proportion of reaction-retarded material in the quicklime. ZKG Int 64:61–72
Ferraz E, Gamelas JAF, Coroado J, Monteiro C, Rocha F (2018) Recycling waste seashells to produce calcitic lime: characterization and wet slaking reactivity. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0232-y
Adams FW (1927) Effect of particle size on the hydration of lime. Ind Eng Chem 19:589–591
Tadros ME, Skalny J, Kalyoncu RS (1976) Kinetics of calcium hydroxide crystal growth from solution. J Colloid Interface Sci 55:20–24
Ritchie IM, Xu B-A (1990) The kinetics of lime slaking. Hydrometallurgy 23:377–396
Giles DE, Ritchie IM, Xu B-A (1993) The kinetics of dissolution of slaked lime. Hydrometallurgy 32:119–128
Wolter A, Luger S, Schaefer G (2004) The kinetics of the hydration of quicklime. ZKG Int 57:60–68
Rodriguez-Navarro C, Ruiz-Agudo E, Ortega-Huertas M, Hansen E (2005) Nanostructure and irreversible colloidal behavior of Ca(OH)2: implications in cultural heritage conservation. Langmuir 21:10948–10957
Kemperl J, Maček J (2009) Precipitation of calcium carbonate from hydrated lime of variable reactivity, granulation and optical properties. Int J Miner Process 93:84–88
FAO (2017) http://www.fao.org/faostat/en/#data
García-García G, Woolley E, Rahimifard S, Colwill J, White R, Needham LA (2017) Methodology for sustainable management of food waste. Waste Biomass Valoriz 8:2209–2227
Regulation EC No 1069/2009 of the European Parliament and of the Council of 21 October 2009. Off. J. Eur. Union, L 300/1–33 (2009)
Soares MA, Quina MM, Quinta-Ferreira RM (2013) Co-composting of eggshell waste in self-heating reactors: monitoring and end product quality. Bioresour Technol 148:293–301
Gunasekaran S, Anbalagan G, Pandi S (2006) Raman and infrared spectra of carbonates of calcite structure. J Raman Spectrosc 37:892–899
Tsai WT, Yang JM, Lai CW, Cheng YH, Lin CC, Yeh CW (2006) Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour Technol 97:488–493
Prabakaran K, Balamurugan A, Rajeswari S (2005) Development of calcium phosphate based apatite from hen’s eggshell. Bull Mater Sci 28:115–119
Urmos J, Sharma SK, Mackenzie FT (1991) Characterization of some biogenic carbonates with Raman spectroscopy. Am Miner 76:641–646
Potgieter JH, Potgieter SS, de Waal D (2003) An empirical study of factors influencing lime slaking Part II: lime constituents and water composition. Water SA 29:157–160
Boynton RS (1980) Chemistry and technology of lime and limestone, 2nd edn. Wiley, New York
Montes-Hernandez G, Concha-Lozanoa N, Renarda F, Quirico E (2009) Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: applied and fundamental aspects. J Hazard Mater 166:788–795
Biscontin G, Birelli MP, Zendri E (2002) Characterization of binders employed in the manufacture of Venetian historical mortars. J Cult Herit 3:31–37
Agra CEAS Consulting (2004) Study on the socio-economic implications of the various systems to keep laying hens. Final Report for The European Commission
Acknowledgements
The authors are grateful to Derovo - Derivados de Ovos, S.A. (Pombal, Portugal) and VAC Minerais, S.A. (Rio Maior, Portugal) for the supply of industrial chicken eggshell waste and commercial limestone, respectively. This research was supported by Sistema de Incentivos à Investigação e Desenvolvimento Tecnológico through Quadro de Referência Estratégica Nacional (QREN) and by Geobiotec R&D Unit (UID/GEO/04035/2013) financed by the Fundação para a Ciência e a Tecnologia (FCT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Ferraz, E., Gamelas, J.A.F., Coroado, J. et al. Eggshell waste to produce building lime: calcium oxide reactivity, industrial, environmental and economic implications. Mater Struct 51, 115 (2018). https://doi.org/10.1617/s11527-018-1243-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-018-1243-7