Abstracts
The objective of this study was to investigate the formation and forming mechanism of the related substance E in potassium clavulanate production. The impurity with retention time of 11.1 min in potassium clavulanate final product was confirmed as the related substance E by high performance liquid chromatography with tandem mass spectrometric detection (HPLC-MS/MS).The related substance E analysis during the production of clavulanic acid showed that this impurity could be formed during both the fermentation and purification processes, especially in the later fermentation stage, filtration concentration and back-extraction procedure. Clavulanic acid was the precursor of the related substance E. Studies on its forming mechanism showed that the related substance E was formed by the combination of the imino group of one molecule of clavulanic acid with the carboxyl group of another molecule of clavulanic acid with the opening of β-lactam ring. Results of a multi-factor orthogonal test confirmed that the concentration of clavulanic acid was the dominant factor to accelerate the reaction, while the temperature was another contributing factor. The pH 5.0-6.5 had little impact on the generation of the related substance E.
Potassium clavulanate/production; Clavulanic acid/formation of the Related substance E; Related substance E/formation; Related substance E/forming mechanism High performance liquid chromatography/biotechnological process
O objetivo deste estudo foi investigar a formação da substância E e o respectivo mecanismo na produção de clavulanato de potássio. Confirmou-se a impureza com tempo de retenção de 11,1 min no produto final, clavulanato de potássio, como substância E, por meio de cromatografia líquida de alta eficiência, em conjunto com detecção por espectrometria de massas (CLAE-MS-MS). A análise da substância relacionada E durante a produção do ácido clavulânico mostrou que essa impureza pode ser formada tanto durante a fermentação quanto durante os processos de purificação, especialmente no estágio final de fermentação, filtração, concentração e procedimento de extração. O ácido clavulãnico foi o precursor da substância E. Estudos no mecanismo de sua formação mostraram que a substância E formou-se pela combinação do grupo imina da molécula do ácido clavulânico com o grupo carboxílico de outra molécula de ácido clavulânico, com a abertura do anel β-lactâmico. Resultados do teste ortogonal multifatorial confirmaram que a concentração do ácido clavulânico foi o fator dominante para acelerar a reação, enquanto a temperatura foi outro fator que contribuiu. O pH de 5,0 a 6,5 teve pouco impacto na geração da substância E.
Clavulanato de potássio/produção; Ácido clavulânico/formação da substância E relacionada; Substância E relacionada/mecanismo de formação Cromatografia líquida de alta eficiência/processo biotecnológico
INTRODUCTION
Clavulanic acid, one of the lactamase inhibitors, is a secondary metabolite produced by Streptomyces clavuligerus (Shetty et al., 2010SHETTY, J.; NEETHA, V.M.; PRATAP, K.; ASHA, K. Antibiotic prophylaxis for hysterectomy and cesarean section: amoxicillin-clavulanic acid versus cefazolin. J. Obstet. Gynaecol., v.60, p.419-423, 2010.; Saudagar, Singhal, 2007SAUDAGAR, S.P.; SINGHAL, S.R. A statistical approach using L25 orthogonal array method to study fermentative production of clavulanic acid by Streptomyces clavuligerus MTCC 1142. Appl. Biochem. Biotechnol., v.136, p.345-359, 2007.).It has been shown that clavulanic acid has antibacterial activity against a wide variety of aerobic and anaerobic bacteria (Silva et al., 2009SILVA, C.S.; BOVAROTTI, E.; RODRIGUES, M.I.; HOKKA, C.O.; BARBOZA, M. Evaluation of the effects of the parameters involved in the purification of clavulanic acid from fermentation broth by aqueous two-phase systems. Bioprocess Biosyst. Eng., v.32, p.625-632, 2009.; Dinceret al., 2004DINCER, I.; ERGIN, A.; KOCAGOZ, T. The vitro efficacy of beta-lactam and beta-lactamase inhibitors against multidrug resistant clinical strains of Mycobacterium tuberculosis. Int. J. Antimicrob. Agents, v.23, p.408-411, 2004.). Several methods such as micellelectrokinetic capillary chromatography (MEKC), high performance liquid chromatography (HPLC), and potentiometric methods are used to determine the concentration of clavulanic acid. Studies showed that clavulanic acid has chemical instability and is degraded rapidly into complexes including multiple components (Parag, 2008PARAG, S.; SAUDAGAR, S.A.; SURVASE, R.; SINGHAL, S. Clavulanic acid: a review. Biotechnol. Adv., v.26, p.335-351, 2008.; Sunderland, Vahdat, 2009SUNDERLAND, B.; VAHDAT, L. The influence of potassium clavulanate on the rate of amoxicillin sodium degradation in phosphate and acetate buffers in the liquid state. Drug. Dev. Ind. Pharm., v.35, p.471-479, 2009.). Seven related substances (assigned as A-G, respectively) have been found existing in potassium clavulanate as noted in European Pharmacopoeia, among which the related substance A, B, C, D, and F were formed by the catabolites of clavulanic acid under acid or alkali conditions (Malcolm, 1984MALCOLM, J.F.; MICHAEL, A.; HARRIS, E.H.; ISKANDER, I.Z. Studies on the hydrolysis of clavulanic acid. J. Chem. Soc. Perkin. Trans., v.1, p.1345-1349, 1984.; Haginaka, 1985HAGINAKA, J.; YASUDA, H.; UNO, T.; NAKAGAWA, T. Degradation of clavulanic acid in aqueous alkaline solution: isolation and structural investigation of degradation products. Chem. Pharm. Bull., v.33, p.218-224, 1985.). The related substance G was produced by L-tyrosine and succinate, the precursors, catalyzed by a series of extracellular enzymes. The related substance E was one of the main impurities in the commercially available potassium clavulanate (Zhong et al., 2008).So far there has been little relevant research on the related substance E and its forming mechanism. In this study, the impurity with retention time of 11.1 min in potassium clavulanate was confirmed as the related substance E by HPLC-MS/MS and the forming mechanism of the related substance E was investigated according to its chemical structure. The effect of varied reaction parameters on the formation of the related substance E was studied, which would be useful to reduce the concentration of the related substance E and improve the quality of potassium clavulanate.
MATERIAL AND METHODS
Microbiol strains
The industrial production strain was Streptomyces clavuligeru F613-1.
Reagents
Ammonium formate, formic acid and methanol were products of Tedia, United States of America with LC grade. Ultra-pure water used for the preparation of running buffers and standard solutions were obtained by a Milli-Q system. All other reagents such as acetone, isopropyl alcohol, ethanol, acetic acid, sodium hydroxide were of analytical reagent grade, which were produced by Sinopharm Chemical Reagent Co. Ltd.
Standard and preparations
Clavulanate lithium standard, crude product and high purity of clavulanate tert-butylamine, potassium clavulanate and industrial fermentation medium were provided by Lunan Pharmaceutical Co. (Shandong, China).
Instrumental parameters
Agilent 1100 HPLC and Agilent 1200-6410 LC-MS/MS systems were used to determine the concentration and structure of the related substance E. A Mettler AL104 electronic analytical balance was used to weigh the sample. A Thermostat water bath and DLSB low temperature bath was used to control the temperature in the orthogonal experiment.
HPLC analysis conditions
Mobile phase A was 0.03 mol/L ammonium formate solution (pH 4), and mobile phase B was an equivalent mixture of methanol and mobile phase A. The elution gradient was 0-4 min mobile phase A 100%, 4-15 min mobile phase B 0-50%, 15-18 min mobile phase B 50-0%, 18-30 min mobile phase A 100% and the flow rate was 1 mL/min. The column temperature inside the column was maintained at 40 °C. Detection was carried out at 230 nm. The injection volume was 20 µL. Samples were well separated on an inertsil ODS-3 chromatographic column (4.6 mm×150 mm, 5 µm).
HPLC-MS/MS analysis conditions
HPLC analysis conditions were the same as above with post-column split, electrospray ionization, negative ion, MS2 scan. The collision energy was 5 V.
The related substance E analysis during the production of clavulanic acid
The production process of clavulanic acid consisted of fermentation, filtration concentration(microfiltration, ultra-filtration, reverse osmosis), extraction, back-extraction, crystallization (crude product) and re-crystallization (final product). Blank medium (sterilized) and the fermentation liquids of different periods (24 h, 48 h, 72 h and 96 h, respectively) during the fermentation process were sampled, and the filtration concentrate, extraction phase, back-extraction concentrate, crude product and final product during the purification process were also collected. All the samples were treated with the following methods according to their characteristics and the concentration of clavulanic acid, and the related substance E was determined by HPLC, respectively.
Samples were obtained as follows. Precisely 5 g of fermentation liquid was weighed and diluted to 25 mL with mobile phase A. The aqueous phase was collected after centrifugation (at a speed of 8000 rpm for 4 min) and filtered (with 0.22 μm membrane). As for the aqueous samples, they were diluted to a final concentration of 1.0 mg/mL clavulanic acid. As for the samples in organic phase, they were blown dry with nitrogen, then dissolved and diluted with mobile phase A. As for the solid samples, precisely 0.25 g was weighed and then diluted into 25 mL with mobile phase A.
Studies on the factors affecting the formation of the related substance E
Clavulanate tert-butylamine of 99.5% purity was prepared with the following procedure: the crude product was weighed and solubilized in 4 °C with ultra-pure water, then adjusted to pH 6.2 with acetic acid. The solution was filtered with a 0.22 μm membrane and then the sample was crystallized in acetone. The crystal was re-dissolved in ultra-pure water, re-crystallized with ethanol and then the crystal was washed with isopropanol and acetone successively. The purity of the crystal was 99.5% or above when determined by HPLC, while the related substance E was not detected.
A three-factor and four-level orthogonal experiment was designed with such three factors as concentration of clavulanic acid, temperature, and pH. Four levels for each factor were set: 50, 100, 150, and 200 g/L for the concentration of clavulanic acid; 5, 10, 15, and 20 °C for temperature, 5.0, 5.5, 6.0 and 6.5 for pH. Appropriate amounts of clavulanate tert-butylamine with 99.5% purity were weighed and dissolved in ultra-pure water. The orthogonal experiment was performed according to the above design, and the changes in concentration of clavulanic acid and the related substance E in 120 min were determined. The effect of the oxygen on the forming velocity of the related substance E under the conditions of air or nitrogen protection at the level of 100 g/L concentration, 15 °C and pH 6.0, was also investigated.
RESULTS AND DISCUSSION
The structure identification of the impurity at 11.1 min
An impurity appeared at the retention time of 11.1 min in some final products of potassium clavulanate. The maximum concentration of the impurity may reach 0.5% (Figure 1). HPLC analysis showed that the impurity is a single substance and the retention time was similar to that of the related substance E as noted in the part of potassium clavulanate in European Pharmacopoeia (2005). So we speculated that the impurity was the related substance E (Figure 2).
HPLC chromatogram of clavulanic acid and the related substance E. A: clavulanic acid; B: the related substance E.
HPLC is a valid method for the determination of free arginine, glutamine,and β-alanine in nutritional products and dietary supplements (Jeffrey, Paul, 2012JEFFREY, H.B.; PAUL, W.J. Determination of free arginine, glutamine, and β-alanine in nutritional products and dietary supplements. Food Anal. Methods, v.5, p.821-827, 2012.). HPLC-MS/MS is also a method to analyse tebuconazole, tetraconazole enantiomers and plant growth regulators (Ma et al., 2013; Zhang et al., 2012ZHANG, H.; QIAN, M.R.; WANG, X.Q.; WANG, X.Y.; XU, H.; QI, P.P.; WANG, Q.; WANG, M.H. Analysis of tebuconazole and tetraconazole enantiomers by chiral HPLC-MS/MS and application to measure enantio selective degradation in strawberries. Food Anal. Methods, v.5, p.1342-1348, 2012.). Tandem mass spectrometry analysis was executed by HPLC-MS/MS in order to define the exact chemical structure of the related substance E. First-order and secondary mass spectrogram were shown in Figure 3a and Figure 3b, respectively. Results of the mass spectrometry showed its molecular ion mass-to-charge ratio was m/z 397, the same as that of the related substance E in European Pharmacopoeia. All the data showed that the impurity was the related substance E.
Mass spectra of the substance with retention time of 11.1 min. a. First-order mass spectrum; b. Secondary mass spectrum.
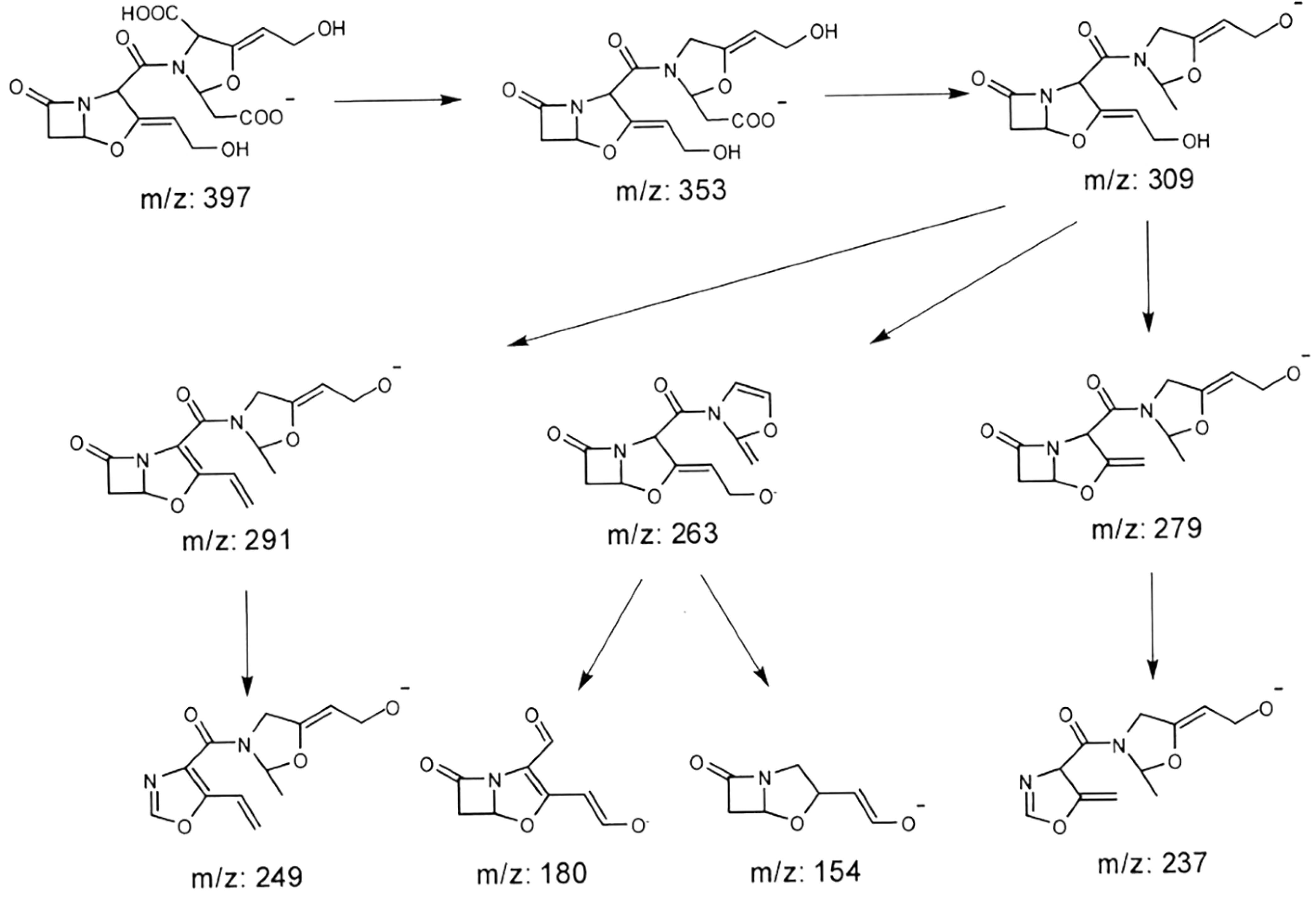
Tandem mass spectrometry analysis showed that a molecular ion with m/z 397 could be dissociated into such fragments as m/z 353, m/z 309, m/z 291, m/z 279, m/z 263, m/z 253, m/z 249, m/z 237, m/z 221, m/z 180, and m/z 154. Combining the characteristics of fragment ions, relative abundance, the general rule of ion fragmentation mechanism, and the chemical structure of the related substance E, we speculated the possible fragmentation path of molecular ion with m/z 397 (Figure 4).
Analysis of the producing stage of the related substance E
The changes in concentration of the related substance E during the fermentation and purification process were studied in order to analyse the possible synthesis pathway of the related substance E. The concentrations of the related substance E at the time of 0 h, 24 h, 48 h, 72 h, and 96 h during fermentation process are shown in Figure 5. There was neither the related substance E nor clavulanic acid in the sterilized blank medium. Approximately 0.3 g/L clavulanic acid was detected at the 24 h stage of fermentation, while none of the related substance E was detected. The concentration of the clavulanic acid reached 2.07 g/L at the stage of 48 h, when the related substance E was 0.0004 g/L. After this time, the concentrations of clavulanic acid and the related substance E increased synchronously. The concentration of clavulanic acid and the related substance E reached 4.87 g/L and 0.044 g/L respectively when fermentation terminated, and the relative amount of the related substance E to clavulanic acid was 0.91%. Results showed that the formation of the related substance E was later than the synthesis of clavulanic acid, and its concentration increased with the increase of clavulanic acid, which was different from the phenomenon that the related substance G was formed almost synchronously with clavulanic acid during the fermentation process.
Concentration of the related substance E during fermentation process. A:clavulanic acid, B:the related substance E; 1:Sterilized blank medium (0h); 2:Early stage of fermentation (24h); 3:Medium stage of fermentation (48h); 4:Later stage of fermentation (72h); 5:Final stage of fermentation (96h)
During the purification process, the relative amount of the related substance E was detected at each procedure (Figure 6). The relative amount of the related substance E was 0.91% at the final fermentation stage, and it decreased to 0.63%, 0.34%, 0.16%, respectively in extraction phase, crude product, and final product. All the above three processes could remove most of the related substance E, while the relative amount of the related substance E increased significantly in filtration concentrate and back-extraction concentrate compared with the corresponding previous process, fermentation and extraction, with a great increase of 21.98% and 36.51%, respectively. So the related substance E may be formed during the concentration stage, which may be related to the increase of clavulanic acid. Considering the formation of the related substance E during the fermentation stage, we could confirm that the related substance E was formed during the fermentation and concentration process, which was related to the increase of clavulanic acid. Combined with the chemical structure of the related substance E, we could deduce that clavulanic acid is the precursor of the related substance E.
The relative percentage amount of the related substance E during the purification process. A: Fermentation broth; B: Filtration concentrate; C: Extraction phase; D: Reversed concentrate; E: Crude salt; F: Final product.
Inference for the forming mechanism of the related substance E
With the analysis of the chemical structure of the related substance E (Figure 2), we find that the molecular weight of the related substance E was twice that of clavulanic acid. We could infer from the formula that the related substance E would be formed from one molecule of clavulanic acid and one molecule of its β-lactam ring opening decomposition product. So the related substance E may be synthesized from two molecules of clavulanic acid. We prepared a 100 g/L clavulanate tert-butylamine solution of 99.5% purity at room temperature in order to verify whether enzyme was required for the production of the related substance E. Results showed that the content of the related substance E increased with prolonged time (Table I). So, the related substance E could be synthesized in a chemical process and the related substance E may be formed in the same way during the fermentation and purification process.
Levels of the orthogonal design on the factors affecting the synthesis of the related substance E
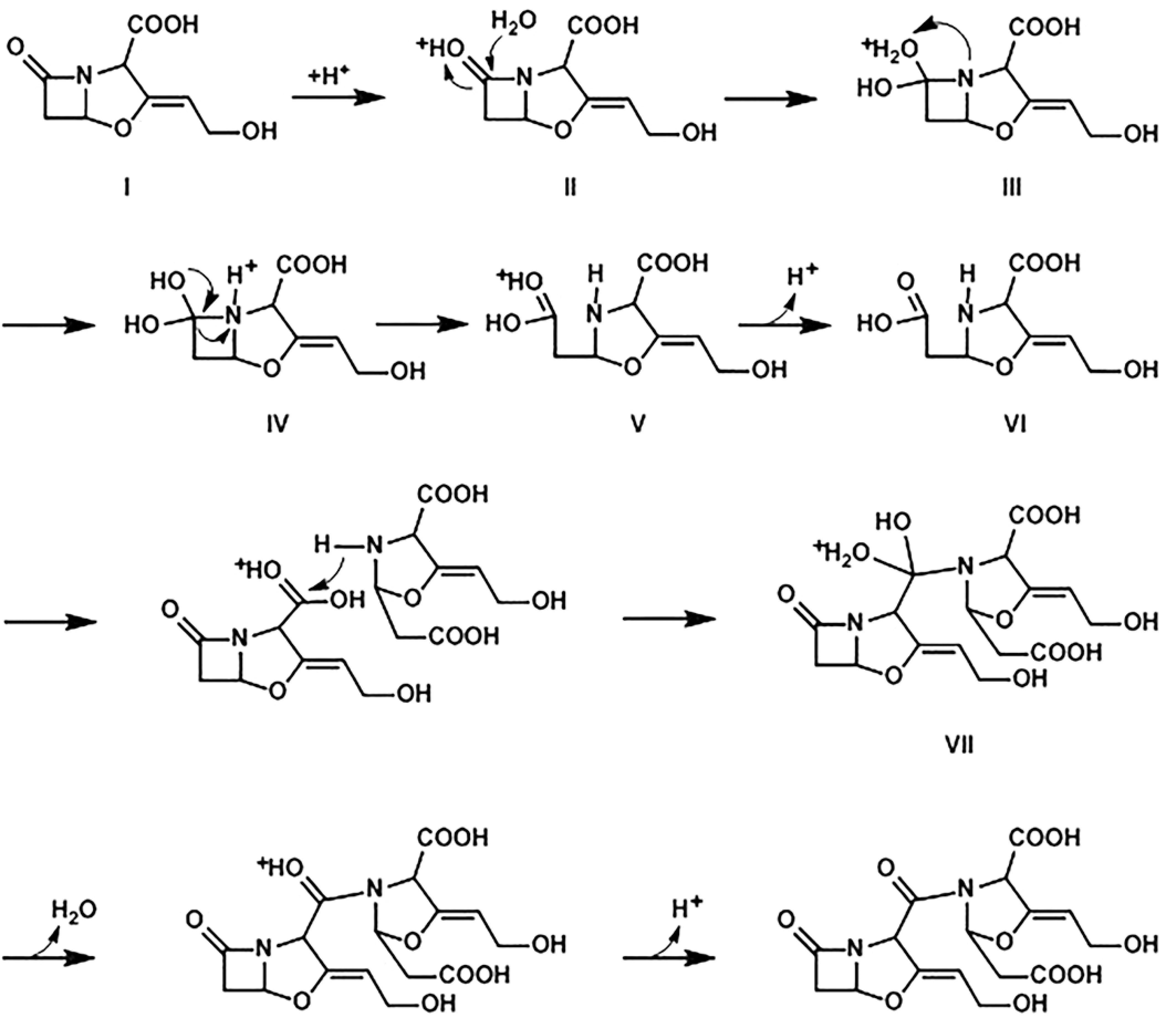
Clavulanic acid had similar chemical properties as other β-lactam antibiotics, and the instability of clavulanic acid was ten times that of penicillin (McGowan et al., 1998MCGOWAN, S.J.; BYCROFT, B.W.; SALMOND, G.P. Bacterial production of carbapenems and clavams: evolution of beta-lactam antibiotic pathways. Trends Microbiol., v.6, p.203-208, 1998.).When the β-lactam ring of clavulanic acid reacted with water or other nucleophilic groups, the hydrolysis of the amide bond may lead to the opening of the β-lactam ring, resulting in the degradation of clavulanic acid (Malcolm, 1984MALCOLM, J.F.; MICHAEL, A.; HARRIS, E.H.; ISKANDER, I.Z. Studies on the hydrolysis of clavulanic acid. J. Chem. Soc. Perkin. Trans., v.1, p.1345-1349, 1984.). The forming mechanism of the related substance E may be described in Figure 7 as follows: 1) in weak acidic conditions, acyl groups in β-lactam ring of clavulanic acid molecule (I) may be oxygen protonated, with the result that the carbonyl carbon is more susceptible to nucleophilic attack; 2) such weak nucleophiles as water attack protonated carbonyl carbon to form the intermediate product (III); 3) the proton would be transferred to a tertiary N, when a lone pair of electrons separated themselves from -OH leading to the opening of the β-lactam ring to form oxonium salt (V); 4) a proton is separated from the oxonium salt in order to form the intermediate product (VI); 5) the imino group of the intermediate product (VI) is activated when being attacked by water, the protonated carboxyl carbon of another molecule of clavulanic acid is attacked by the resulting active nitrogen, and oxonium salt is formed(VIII); 6) the proton is separated from the oxonium salt and the amide bond formed, leading that the related substance E (IX) formed. The above specific reaction route is shown in Figure 7.
Factors affecting the synthesis of the related substance E
The main controlling parameters during the production of clavulanic acid were the concentration of clavulanic acid, pH, and the temperature. We designed an orthogonal test to study their effect on the synthesis of the related substance E. The orthogonal design and the results are listed in Table I and Table II, respectively.
Results of the orthogonal design on the factors affecting synthesis of the related substance E
Range analysis showed that various factors affected the formation of the related substance E. The influence order was concentration of clavulanic acid> temperature >pH. Both concentration and temperature were significant in α=0.05 level. So we confirmed that they were the main factors affecting the synthesis of the related substance E, while the effect of pH was not obvious.
Results of the orthogonal experiment showed that the related substance E increased obviously when the concentration of clavulanic acid reached 150 g/L, which illustrated that the synthesis of the related substance E conformed to the law of mass action. As the content of the substrates increases, the probability of the collision among clavulanic acid molecules and their loop-opening degradation products is magnified and a new amide bond is generated. However, when the clavulanic acid concentration decreases, the synthesis of the related substance E decreases correspondingly, which may be the reason that the concentration of the related substance E increases obviously during the final fermentation.
Temperature was another factor affecting the synthesis of the related substance E. In general, the amide bond is relatively stable for the delocalization of the lone electron pair from the nitrogen atom itself, so it is prone to being hydrolysed under strong acid, alkali or heating conditions. Results showed that there were still trace amide bonds of clavulanic acid molecules breaking with the ring opened. The cleavage of the amide bond needs energy provided by the system. So the concentration of the related substance E decreased obviously at 5-10 °C, while the concentration increased significantly at 20 °C, during the orthogonal experiment, which proved that high temperature was favorable to provide energy for ring opening reaction. This may be the reason why the related substance E would be accumulated during the fermentation, although the concentration of clavulanic acid was low (0-4.87 g/L), but it was easier for the related substance E to form when the temperature is maintained at 28 °C.
Many studies showed that pH affected the stability of clavulanic acid, which was the vital factor to cause hydrolysis of clavulanic acid (Malcolm, 1984MALCOLM, J.F.; MICHAEL, A.; HARRIS, E.H.; ISKANDER, I.Z. Studies on the hydrolysis of clavulanic acid. J. Chem. Soc. Perkin. Trans., v.1, p.1345-1349, 1984.; Haginaka et al., 1985HAGINAKA, J.; YASUDA, H.; UNO, T.; NAKAGAWA, T. Degradation of clavulanic acid in aqueous alkaline solution: isolation and structural investigation of degradation products. Chem. Pharm. Bull., v.33, p.218-224, 1985.; Martin et al., 1989MARTIN, J.; MENDEZ, R.; ALEMANY, T. Studies on clavulanic acid. Part 1. Stability of clavulanic acid in aqueous solutions of amines containing hydroxy groups . J. Chem. Soc. Perkin. Trans., v.2, p.223-226, 1989.). Our results showed that pH had little impact on the formation of the related substance E in the pH range of 5.0-6.5, which was consistent with the fact that maintenance of pH 5.0-6.5 was beneficial to improve the quality of potassium clavulanate. In addition, the forming velocity of the related substance E was studied at nitrogen protection or exposure to air conditions. Although the higher productivity of secondary metabolites was attainable through improving the oxygen supply during the culture process (Dick et al., 1994DICK, O.; ONKEN, U.; SATTER, I.; ZEECK, A. Influence of increased dissolved oxygen concentration on productivity and selectivity in cultures of a colabomycin-producing strain of Streptomyces griseo-avus. Appl. Microbiol. Biotechnol., v.41, p.373-377, 1994.; Kaiser et al., 1994KAISER, D.; ONKEN, U.; SATTLER, I.; ZEECK, A. Influence of increased dissolved oxygen concentration on the formation of secondary metabolites by manumycin-producing Streptomyces parvulus. Appl. Microbiol. Biotechnol., v.41, p.309-312, 1994.), Kim verified that active oxygen(s) could exert control on the expression of secondary metabolite biosynthesis (Kwon, Kim, 1999KWON, H.J.; KIM, S.U. Molecular basis for enhanced biosynthesis of clavulanic acid by a redox-cycling agent, phenazinemetho sulfate, in Streptomyces clavuligerus. Appl. Microbiol. Biotechnol., v.53, p.57-62, 1999.). In this study, oxygen had little effect on the generation of the related substance E (Table II).
Back extraction process conditions were optimized in according to the results of this study. The concentration of the related substance E decreased from an average of 0.18% to 0.03% with the decrease of clavulanic acid 150 g/L to 100 g/L. The reaction temperature was adjusted from 15 °C to 5 °C, and pH was maintained at 5.5, which showed that the concentration of clavulanic acid and temperature are strongly correlated to the related substance E synthesis.
CONCLUSIONS
HPLC-MS/MS analysis proved the impurity with a retention time of 11.1 min is the related substance E, as noted in European Pharmacopoeia (2005). The related substance E is significantly formed during the concentration process after synthesis of clavulanic acid in the fermentation.
The forming mechanism of the related substance E involves the following steps: 1. a water attack to the molecule of clavulanic acid; 2. the opening of β-lactam ring amide bond, forming an amino group and a carboxylic group; 3. reaction of the amino group from a clavulanic acid molecule with the carboxylic group of another clavulanic acid molecule to form a new amide bond, generating the related substance E at the same time.
Analysis of an orthogonal experiment showed that the concentration of clavulanic acid was the vital factor to promote the generation of the related substance E, while the temperature was another contributing factor. The pH had little impact on the generation of the related substance E in the range of 5.0-6.5.
ACKNOWLEDGEMENTS
We thank Dr. Jiang Nan from the Chinese Academy of Sciences, who gave us valuable advice about the generation mechanism of the related substance E.
REFERENCES
- DICK, O.; ONKEN, U.; SATTER, I.; ZEECK, A. Influence of increased dissolved oxygen concentration on productivity and selectivity in cultures of a colabomycin-producing strain of Streptomyces griseo-avus. Appl. Microbiol. Biotechnol., v.41, p.373-377, 1994.
- DINCER, I.; ERGIN, A.; KOCAGOZ, T. The vitro efficacy of beta-lactam and beta-lactamase inhibitors against multidrug resistant clinical strains of Mycobacterium tuberculosis. Int. J. Antimicrob. Agents, v.23, p.408-411, 2004.
- EUROPEAN Pharmacopoeia Commission of the Council of Europe. Ph. Eur. 5.0, p.22278-22279, 2005.
- HAGINAKA, J.; YASUDA, H.; UNO, T.; NAKAGAWA, T. Degradation of clavulanic acid in aqueous alkaline solution: isolation and structural investigation of degradation products. Chem. Pharm. Bull., v.33, p.218-224, 1985.
- JEFFREY, H.B.; PAUL, W.J. Determination of free arginine, glutamine, and β-alanine in nutritional products and dietary supplements. Food Anal. Methods, v.5, p.821-827, 2012.
- KAISER, D.; ONKEN, U.; SATTLER, I.; ZEECK, A. Influence of increased dissolved oxygen concentration on the formation of secondary metabolites by manumycin-producing Streptomyces parvulus. Appl. Microbiol. Biotechnol., v.41, p.309-312, 1994.
- KWON, H.J.; KIM, S.U. Molecular basis for enhanced biosynthesis of clavulanic acid by a redox-cycling agent, phenazinemetho sulfate, in Streptomyces clavuligerus. Appl. Microbiol. Biotechnol., v.53, p.57-62, 1999.
- MA, L.Y.; ZHANG, H.Y.; XU, W.T.; HE, X.Y.; YANG, L.L.; LUO, Y.B.; HUANG, K.L. Simultaneous determination of 15plant growth regulators in bean sprout and tomato with liquid chromatography-triple quadrupole tandem mass spectrometry. Food Anal. Methods, v.6, p.941-951, 2013.
- MALCOLM, J.F.; MICHAEL, A.; HARRIS, E.H.; ISKANDER, I.Z. Studies on the hydrolysis of clavulanic acid. J. Chem. Soc. Perkin. Trans., v.1, p.1345-1349, 1984.
- MARTIN, J.; MENDEZ, R.; ALEMANY, T. Studies on clavulanic acid. Part 1. Stability of clavulanic acid in aqueous solutions of amines containing hydroxy groups . J. Chem. Soc. Perkin. Trans., v.2, p.223-226, 1989.
- MCGOWAN, S.J.; BYCROFT, B.W.; SALMOND, G.P. Bacterial production of carbapenems and clavams: evolution of beta-lactam antibiotic pathways. Trends Microbiol., v.6, p.203-208, 1998.
- PARAG, S.; SAUDAGAR, S.A.; SURVASE, R.; SINGHAL, S. Clavulanic acid: a review. Biotechnol. Adv., v.26, p.335-351, 2008.
- SAUDAGAR, S.P.; SINGHAL, S.R. A statistical approach using L25 orthogonal array method to study fermentative production of clavulanic acid by Streptomyces clavuligerus MTCC 1142. Appl. Biochem. Biotechnol., v.136, p.345-359, 2007.
- SHETTY, J.; NEETHA, V.M.; PRATAP, K.; ASHA, K. Antibiotic prophylaxis for hysterectomy and cesarean section: amoxicillin-clavulanic acid versus cefazolin. J. Obstet. Gynaecol., v.60, p.419-423, 2010.
- SILVA, C.S.; BOVAROTTI, E.; RODRIGUES, M.I.; HOKKA, C.O.; BARBOZA, M. Evaluation of the effects of the parameters involved in the purification of clavulanic acid from fermentation broth by aqueous two-phase systems. Bioprocess Biosyst. Eng., v.32, p.625-632, 2009.
- SUNDERLAND, B.; VAHDAT, L. The influence of potassium clavulanate on the rate of amoxicillin sodium degradation in phosphate and acetate buffers in the liquid state. Drug. Dev. Ind. Pharm., v.35, p.471-479, 2009.
- ZHANG, H.; QIAN, M.R.; WANG, X.Q.; WANG, X.Y.; XU, H.; QI, P.P.; WANG, Q.; WANG, M.H. Analysis of tebuconazole and tetraconazole enantiomers by chiral HPLC-MS/MS and application to measure enantio selective degradation in strawberries. Food Anal. Methods, v.5, p.1342-1348, 2012.
- ZHONG, C.Q.; CAO, G.X.; CHEN, F. Determination of the related substances in clavulanate potassium by HPLC/ESI/MS. Chin. J. Antibiot., v.33, p.750-752, 2008.
Publication Dates
-
Publication in this collection
Apr-Jun 2014
History
-
Received
05 Sept 2013 -
Accepted
12 Nov 2013