Abstracts
Most of the problems of endodontic origin have a bacterial etiological agent. Thus, there is a continued interest in seeking more effective chemical substances that can replace the camphorated paramonochiorophenol or antibiotics as intracanal medicaments. Among the possible substances, ozone has some interesting biological characteristics: bactericidal action, debriding effect, angiogenesis stimulation capacity and high oxidizing power. The purpose of this study was to chemically evaluate the presence of ozone in sunflower, castor, olive and almond oil, as well as in propylene glycol and byproducts of ozonation, such as formaldehyde. These compounds were ozonized, inserted into empty and sterile vials, and analyzed by testing the reaction between ozone and indigo, for determining the presence of ozone, and subjected to the chromotropic acid test for determining the presence of formaldehyde. It was observed complete absence of ozone in all samples tested and presence of formaldehyde. The bactericidal and healing action of ozonized oils could be attributed to products formed by the ozonation of mineral oils, such as formaldehyde, not to the ozone itself.
Endodontics; ozone; formaldehyde
A maioria dos problemas de origem endodôntica tem um agente etiológico bacteriano. Assim, existe um interesse permanente em se buscar substâncias químicas mais efetivas e que possam substituir o PMCC ou os antibióticos como curativos de demora. Dentre as possíveis substâncias, o ozônio apresenta algumas características biológicas interessantes: ação bactericida, efeito debridante, estímulo a angiogênese, além do efeito oxidante. O propósito do presente estudo foi avaliar quimicamente a presença de ozônio nos óleos de girassol, rícino, oliva e amêndoas, além do propilenoglicol, bem como subproputos da ozonização, como formaldeído. Essas substâncias foram ozonizadas, inseridas em tubetes anestésicos vazios e esterilizados, e analisadas por meio do teste da reação entre ozônio e índigo, para determinação da presença de ozônio; e teste do ácido cromotrópico, para determinação da presença de formaldeído. Foi observado ausência total de ozônio em todas as amostras testadas, além da presença de formaldeído. A ação bactericida e curativa dos óleos ozonizados poderia ser atribuída aos produtos formados pela ozonização de óleos minerais, como o formaldeído, e não ao ozônio propriamente dito.
Ozonized oils: a qualitative and quantitative analysis
Adriana Simionatto GuinesiI; Carolina AndolfattoI; Idomeo Bonetti FilhoI; Arnaldo Alves CardosoII; Juliano Passaretti FilhoII; Roberta Vieira FaracI
IDiscipline of Endodontics, Department of Restorative Dentistry, Araraquara Dental School, UNESP - Univ. Estadual Paulista, Araraquara, SP, Brazil
IIDiscipline of Analytical Chemistry, Department of Analytical Chemistry, Institute of Chemistry, UNESP - Univ. Estadual Paulista, Araraquara, SP, Brazil
Correspondence Correspondence: Prof. Dr. Idomeo Bonetti Filho, Departamento de Odontologia Restauradora, Faculdade de Odontologia de Araraquara, UNESP, Rua Humaitá, 1680, Caixa Postal 331, 14801-903 Araraquara, SP, Brazil. Tel: +55-16-3301-6388/6393. e-mail: idomeo@foar.unesp.br
ABSTRACT
Most of the problems of endodontic origin have a bacterial etiological agent. Thus, there is a continued interest in seeking more effective chemical substances that can replace the camphorated paramonochiorophenol or antibiotics as intracanal medicaments. Among the possible substances, ozone has some interesting biological characteristics: bactericidal action, debriding effect, angiogenesis stimulation capacity and high oxidizing power. The purpose of this study was to chemically evaluate the presence of ozone in sunflower, castor, olive and almond oil, as well as in propylene glycol and byproducts of ozonation, such as formaldehyde. These compounds were ozonized, inserted into empty and sterile vials, and analyzed by testing the reaction between ozone and indigo, for determining the presence of ozone, and subjected to the chromotropic acid test for determining the presence of formaldehyde. It was observed complete absence of ozone in all samples tested and presence of formaldehyde. The bactericidal and healing action of ozonized oils could be attributed to products formed by the ozonation of mineral oils, such as formaldehyde, not to the ozone itself.
KeyWords: Endodontics, ozone, formaldehyde.
RESUMO
A maioria dos problemas de origem endodôntica tem um agente etiológico bacteriano. Assim, existe um interesse permanente em se buscar substâncias químicas mais efetivas e que possam substituir o PMCC ou os antibióticos como curativos de demora. Dentre as possíveis substâncias, o ozônio apresenta algumas características biológicas interessantes: ação bactericida, efeito debridante, estímulo a angiogênese, além do efeito oxidante. O propósito do presente estudo foi avaliar quimicamente a presença de ozônio nos óleos de girassol, rícino, oliva e amêndoas, além do propilenoglicol, bem como subproputos da ozonização, como formaldeído. Essas substâncias foram ozonizadas, inseridas em tubetes anestésicos vazios e esterilizados, e analisadas por meio do teste da reação entre ozônio e índigo, para determinação da presença de ozônio; e teste do ácido cromotrópico, para determinação da presença de formaldeído. Foi observado ausência total de ozônio em todas as amostras testadas, além da presença de formaldeído. A ação bactericida e curativa dos óleos ozonizados poderia ser atribuída aos produtos formados pela ozonização de óleos minerais, como o formaldeído, e não ao ozônio propriamente dito.
INTRODUCTION
Most of the problems of endodontic origin have a bacterial etiological agent. Although other factors of physical or chemical order may be involved, it is unquestionable that, in most cases, bacteria and their byproducts play a key role in the induction of pulpal and periradicular pathology (1).
As bacteria and their byproducts are not often reached by the biomechanical action of instruments and chemicals, especially those located in the dentinal tubules, apical ramifications, areas of resorption and apical biofilm, the use of an antibacterial intracanal medication is recommended for disinfection of the root canal system in teeth with chronic periapical lesions (2).
There is a continued interest in the search for chemical compounds with broader and more effective action that could substitute with advantage paramonochiorophenol or antibiotics as intracanal medications. Moreover, the persistence of positive cultures after instrumentation, disinfection and use of calcium hydroxide justifies the search for new antimicrobial substances (3). Among the possible substances to be used in Endodontics, ozone has been considered as promising because it has some interesting biological characteristics: bactericidal action, debriding effect, angiogenesis stimulation capacity and high oxidizing power.
In reaction to oils, ozone breaks the double bonds between carbon atoms of lipid molecules, resulting in new stable molecules such as ketones and aldehydes and unstable species, such as hydrogen peroxide, and radicals such as OH (4,5).
The antimicrobial effect of ozone in dentistry is still not very widespread, but it has attracted some researchers interested in studying this subject. This gas has been proven effective against several microbial species found in the oral cavity, and as a method of disinfecting dentures (6).
In Endodontics, studies have focused on the use of ozone as an intracanal medication and as an irrigant agent (7-10). Despite pioneering applications of ozone in treatment of pulpitis and pulp gangrene (11), no consensus has been reached about the experimental results (12). The purpose of this study was to chemically evaluate the presence of dissolved ozone in sunflower, castor, olive and almond oils, as well as in propylene glycol vehicle and byproducts usually formed after reaction with ozone and organic molecules containing double bonds of ozonation, such as formaldehyde.
MATERIAL AND METHODS
In this study were analyzed: sunflower oil, castor oil, olive oil and almond oil (commonly used in medical and dental areas) as well as propylene glycol, which are potentially used in dentistry, including endodontic therapy. Propylene glycol was used as a control, as it is a vehicle for intracanal medication widely used in Endodontics.
In sample preparation, 5 mL of each material were added to 6 test tubes separately. The materials were then subjected to bubbling oxygen for 60 min each, obtained from an ozone generator (model OZONIC C-20, São Bernardo do Campo, SP, Brazil). The supply pressure of medical oxygen was 5 L/min with a power conversion of 1 (maximum power of the generator), which produced a concentration of 9 mg/L. Immediately after ozonation, the materials were inserted into 5 mL Luer syringes, through which ozonized products were placed in autoclaved empty 18-mL glass anesthetic cartridges. The tubes were sealed with rubber seals of their own pre-sterilized vials. After 24 h, the samples were taken to the Laboratory of the Department of Analytical Chemistry of the Institute of Chemistry/UNESP, for appropriate tests.
Analysis of Ozone Presence - Ozone/Indigo Reaction
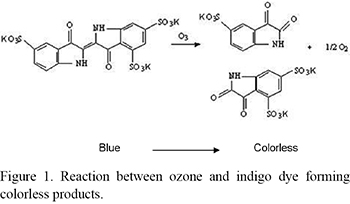
A colorimetric method testing the reaction between ozone and indigo trisulfonic was used for qualitative and quantitative determination of ozone in the samples. This is a very specific method and is based on the fact that when ozone and indigo trisulfonic are in contact a reaction starts and cause breakage of the dye molecule, forming colorless products (13) (Fig. 1). The bleaching of the dye solution is an indicative of the ozone presence (qualitative test). The change in the dye color intensity is proportional to the amount of ozone contained in the solution (quantitative test).
Were added in a glass beaker 5 mL of distilled water and 1 mL of indigo trisulfonic solution 6 mM in phosphate buffer 0.05 M at pH 2.2 . Then, two drops of oil sample treated with ozone were added to this solution. Immediately after that, the color obtained in the mixture was checked. Changing of the color of the solution into blue meant that there was no reaction between ozone and indigo dye, proving the absence of the ozone. Otherwise, if the resulting solution became colorless, it confirmed the presence of ozone, since there was reaction between the ozone and the dye.
Analysis of Formaldehyde Presence - Test with Chromotropic Acid
Formaldehyde may be present as a byproduct of the reaction of lipids and ozone. It is a compound with antibacterial characteristics, but it is toxic and can interfere with cellular integrity. Thus, assessing the presence of formaldehyde becomes indispensable.
When a solution containing formaldehyde is mixed and heated with a solution of chromotropic acid dissolved in concentrated sulfuric acid, the final solution becomes purple. In a capsule of porcelain was added 1 mL of sulfuric acid 98% (w\v) and 2 drops of oil treated with ozone. Then, the sample was taken at a gentle warming hot plate to about 85°C. The solution was left at rest to be cooled and finally were added 4 mL of distilled water. After 10 min of the solution at rest, it was observed if the violet color was formed, indicating the presence of formaldehyde in the solution.
RESULTS
There was no change in the blue color of the solution of the indigo trisulfonic dye, proving the absence of ozone in all samples tested. The test with chromotropic acid showed the formation of the violet dye, confirming the presence of formaldehyde in all samples tested.
DISCUSSION
Ozone has distinct mechanisms of action depending on the environment in which it is applied, being subject to different classifications according to their predominant behavior. Humidity, oxygenation and temperature have a great effect on the action of ozone, being directly proportional to its effectiveness (14,15).
Among the substances that can be used as intracanal medications, ozone has some interesting biological characteristics. Ozone is a very reactive gas; it oxidizes the cell walls and cytoplasmic bacterial membranes, also acting on fungi, protozoa and viruses. These antimicrobial properties can be used to treat several diseases. According to Huth et al. (16), ozone in optimal concentrations is nontoxic to human cells and tissues and has extremely destructive power against bacteria and other microorganisms. However, it should be carefully used, since this gas has unsaturated structures like molecules of fatty acids and protein constituents of biological membranes as the main target, potentially causing damage to all cells of the respiratory tract (17).
Ozone is a gas generated by the passage of oxygen on a high voltage electrical equipment. The fact that the ozone is a gas, makes it use difficult in dental offices. A suggested alternative is to use a liquid to dissolve the gas and use a chemical for its stabilization. Ozone is a highly reactive gas and its main characteristic is to react with molecules that have double bond between carbon atoms (> C = C <), breaking the connection with the introduction of an oxygen atom at each end of the link (2> C = O) and thus forming two new compounds. This property explains its deleterious effects on dyes, rubber and natural organic molecules present in the tissues that form the respiratory tract.
Several of the formulations described as ozone stabilized liquid solutions use vegetable oils such as olive oil. Molecules that composes the vegetable oils have double bond > C = C < in its structure. Therefore, it can be expected that the contact of ozone with these substances generate new compounds by the reaction of breaking the double bonds.
The ozone present in oily vehicles could have advantages over gaseous or aqueous media (6,18), since the oil remains in contact with the surface for a longer period of time, exercising its functions for a longer period. While the half-life of ozone in gaseous form is ephemeral, in the form of oil it allows storage for several months, dispensing the need for a generator.
In this study, several ozonized oils were chemically tested. In reaction to oils, ozone breaks the double bonds between carbon atoms of lipid molecules, resulting in unsaturated molecules that produce different toxic products such as ketones and aldehydes and among these, the formaldehyde (19). In this study, all samples showed absence of ozone. This was observed because the indigo trisulfonic dye when in contact with ozone, has its molecules broken, resulting in the absence of this dye color. In the performed test, there was no change in the solution’s color, which means that there was no reaction between the blue dye and ozone, indicating absence of this gas.
Formaldehyde may be present as a byproduct of lipid and ozone reaction. As formaldehyde is a considerable toxic product and also can interfere with cellular integrity, the evaluation of the presence of formaldehyde has become mandatory. The reaction between formaldehyde and chromotropic acid heated in a solution with concentrated sulfuric acid results in a solution with violet color, indicating the presence of formaldehyde. In the test performed, all samples became violet, showing the presence of formaldehyde in all samples after ozonation.
Propylene glycol was used as control in this study because it is a substance widely used in endodontics as a vehicle for intracanal medication and due to the lack of antimicrobial power (20).
The healing power of the substances evaluated in the present study could be attributed to the ozonized products formed, such as the formaldehyde produced after ozonation, an not the ozone itself. It is noteworthy that some issues on the effect of ozone therapy on the microbiota should be further elucidated, such as the optimal concentration, the penetration of the compound into the dentinal tubules and the ideal application time.
Accepted October 7, 2010
- 1. Leonardo MR, Silveira FF, Silva LAB, Tanomaru Filho M, Utrilla LS. Calcium hydroxide root canal dressing. Histopathological evaluation of periapical repair at different time periods. Braz Dent J 2002;13:17-22.
- 2. Oliveira JCM, Alves FRF, Uzeda M, Rôças IN, Siqueira JrJF. Influence of serum and necrotic soft tissue on the antimicrobial effects of intracanal medicaments. Braz Dent J 2010;21:295-300.
- 3. Estrela C, Estrela CRA, Decurio DA, Hollanda ACB, Silva JA. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine in infected human root canals. Int Endod J 2007;40:85-93.
- 4. Pryor WA, Uppu RM. Akinetic model for the competitive reaction of ozone with amino acid residues in proteins in reverse micelles. J Biol Chem 1993;268:3120-3126.
- 5. Legnani PP, Leoni E, Baraldi M, Pinelli G, Bisbini P. Evaluation of disinfection treatment systems for municipal wastewater reclamation and reuse. Med Microbiol Inf 1996;98:552-566.
- 6. Oizumi M, Suzuki T, Uchida M, Furuya J, Okamoto Y. In vitro testing of a denture cleaning method using ozone. J Med Dent Sci 1998;45:135-139.
- 7. Cruz HFO, Bonetti Filho I, Ampuero BPL. Evaluación in vitro de la asociación del efecto antimicrobiano del ozono unido a vehículos y medicamentos de accion prolongada. Ac Odontol Venez 2008;46:159-164.
- 8. Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M, Nishihara T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol 2004;19: 240-246.
- 9. Nagayoshi M, Kitamura C, Fukuizumi T, Nishihara T, Terashita M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J Endod 2004;30:778-781.
- 10. Huth KC, Quirling M, Maier S, Kamereck K, Alkhayer M, Paschos E, Welsch U, Miethke T, Brand K, Hickel R. Effectiveness of ozone against endodontopathogenic microorganisms. Int Endod J 2009;42:3-13.
- 11. Baradun A, Boitel RH. Thirteen years of experience with the Barandun irrigator and ozone. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endod 1962;15:986-995.
- 12. Cardoso MG, Oliveira LD, Koga-Ito CY, Jorge AO. Effectiveness of ozonated water on Cândida albicans, Enterococcus faecalis, and endotoxins in root canals. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endod 2008;105:85-91.
- 13. Bader H, Hoigné J. Determination of ozone in water by the indigo method. Water Res 1980;15:449-456.
- 14. Nagayoshi M, Fuzuizumi T, Kitamura C, Yano J, Terashita M, Nishihara T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol 2004;19:240-246.
- 15. Ramzy MI, Gomaa HE, Mostafa MI, Zaki BM. Management of aggressive periodontitis using ozonated water. Egypt Med J NRC 2005;6:229-245.
- 16. Huth KC, Jakob FM, Capello C, Saugel B, Paschos E, Hollewenck. Effect of ozone on oral cells compared with established antimicrobials. Eur J Oral Sci 2006;114:435-440.
- 17. Garcia G, Allen AG, Cardoso AA. Development of a sensitive passive sampler using indigotrisulfonate for the determination of tropospheric ozone. J Environ Monit 2010;12:1325-1329.
- 18. Noetzel J, Nonhoff J, Bitter K, Wagner J, Neumman K, Kielbassa AM. Efficacy of calcium hydroxide, Er:YAG laser or gaseous ozone against Enterococcus faecalis in root canals. Am J Dent 2009;22:14-18.
- 19. Díaz MF, Hernández R, Martínez G, Vidal G, Gómez M, Fernández H, et al.. Comparative study of ozonized oil and ozonized sunflower oil. J Braz Chem Soc 2006; 17:403-407.
- 20. Rezende GPSR, Costa LRRS, Pimenta FC, Baroni DA. In vitro antimicrobial activity of endodontic pastes with propolis extracts and calcium hydroxide: A preliminary study. Braz Dent J 2008;19:301-305.
Publication Dates
-
Publication in this collection
20 Apr 2011 -
Date of issue
2011