Abstracts
INTRODUCTION: Surgical planning for refractory medial temporal lobe epilepsy (rMTLE) relies on seizure localization by ictal electroencephalography (EEG). Multiple factors impact the number of seizures recorded. We evaluated whether seizure freedom correlated to the number of seizures recorded, and the related factors. METHODS: We collected data for 32 patients with rMTLE who underwent anterior temporal lobectomy. Primary analysis evaluated number of seizures captured as a predictor of surgical outcome. Subsequent analyses explored factors that may seizure number. RESULTS: Number of seizures recorded did not predict seizure freedom. More seizures were recorded with more days of seizure occurrence (p<0.001), seizure clusters (p<0.011) and poorly localized seizures (PLSz) (p=0.004). Regression modeling showed a trend for subjects with fewer recorded poorly localized seizures to have better surgical outcome (p=0.052). CONCLUSIONS: Total number of recorded seizures does not predict surgical outcome. Patients with more PLSz may have worse outcome.
epilepsy; temporal lobe; epilepsy surgery; electroencephalography
INTRODUÇÃO: O planejamento cirúrgico para epilepsia refratária do lobo medial temporal (rMTLE) depende da localização da região de origem das convulsões por meio do eletroencefalografia (EEG) ictal. Múltiplos fatores podem influenciar o número de crises registradas. Neste artigo, avaliamos se a obtenção de liberdade de crises epilépticas no pós-operatório se relaciona com o número de crises epilépticas registradas durante a avaliação pré-operatória e os fatores que afetam tal resultado. MÉTODOS: Foram coletados dados de 32 pacientes com rMTLE que foram submetidos à lobectomia temporal anterior. A análise principal avaliou o número de convulsões captadas como fator preditivo do resultado cirúrgico, e as análises subsequentes exploraram outros fatores que podem ter afetado o resultado cirúrgico. RESULTADOS: O número de convulsões registradas não mostrou valor preditivo para resultados livres de crises. Foi registrado maior número de convulsões quando houve: maior número de dias em que ocorreram crises (p<0,001); salvas de convulsões (p<0,011); e localização subótima da origem das crises (PLSz) (p=0,004). O modelo de regressão mostrou tendência para os indivíduos com um menor número de crises pobremente localizadas terem um melhor desfecho cirúrgico (p=0,052). CONCLUSÕES: O número total de crises registrado não afeta o desfecho cirúrgico, que possivelmente é influenciado por múltiplos fatores. Pacientes com mais PLSz têm maior possibilidade de pior resultado cirúrgico.
epilepsia; lobo temporal; cirurgia de epilepsia; eletroencefalografia
ARTICLE
Relationship of number of seizures recorded on video-EEG to surgical outcome in refractory medial temporal lobe epilepsy
Relação entre o número de crises epilépticas registradas em vídeo-EEG e o resultado cirúrgico na epilepsia refratária do lobo temporal
Rup Kamal SainjuI; Bethany Jacobs WolfII; Leonardo BonilhaI; Gabriel MartzI
IDivision of Neurology, Department of Neurosciences, Medical University of South Carolina (MUSC), Charleston SC, USA
IIDivision of Biostatistics and Epidemiology, MUSC, Charleston SC, USA
Correspondence Correspondence: Rup Kamal Sainju Division of Neurology, Department of Neurosciences, Medical University of South Carolina 96 Jonathan Lucas St. Suite 307 CSB MSC 606 29425-6060 Charleston SC - USA Email: dr.sainju@gmail.com
ABSTRACT
INTRODUCTION: Surgical planning for refractory medial temporal lobe epilepsy (rMTLE) relies on seizure localization by ictal electroencephalography (EEG). Multiple factors impact the number of seizures recorded. We evaluated whether seizure freedom correlated to the number of seizures recorded, and the related factors.
METHODS: We collected data for 32 patients with rMTLE who underwent anterior temporal lobectomy. Primary analysis evaluated number of seizures captured as a predictor of surgical outcome. Subsequent analyses explored factors that may seizure number.
RESULTS: Number of seizures recorded did not predict seizure freedom. More seizures were recorded with more days of seizure occurrence (p<0.001), seizure clusters (p<0.011) and poorly localized seizures (PLSz) (p=0.004). Regression modeling showed a trend for subjects with fewer recorded poorly localized seizures to have better surgical outcome (p=0.052).
CONCLUSIONS: Total number of recorded seizures does not predict surgical outcome. Patients with more PLSz may have worse outcome.
Key words: epilepsy, temporal lobe, epilepsy surgery, electroencephalography.
RESUMO
INTRODUÇÃO: O planejamento cirúrgico para epilepsia refratária do lobo medial temporal (rMTLE) depende da localização da região de origem das convulsões por meio do eletroencefalografia (EEG) ictal. Múltiplos fatores podem influenciar o número de crises registradas. Neste artigo, avaliamos se a obtenção de liberdade de crises epilépticas no pós-operatório se relaciona com o número de crises epilépticas registradas durante a avaliação pré-operatória e os fatores que afetam tal resultado.
MÉTODOS: Foram coletados dados de 32 pacientes com rMTLE que foram submetidos à lobectomia temporal anterior. A análise principal avaliou o número de convulsões captadas como fator preditivo do resultado cirúrgico, e as análises subsequentes exploraram outros fatores que podem ter afetado o resultado cirúrgico.
RESULTADOS: O número de convulsões registradas não mostrou valor preditivo para resultados livres de crises. Foi registrado maior número de convulsões quando houve: maior número de dias em que ocorreram crises (p<0,001); salvas de convulsões (p<0,011); e localização subótima da origem das crises (PLSz) (p=0,004). O modelo de regressão mostrou tendência para os indivíduos com um menor número de crises pobremente localizadas terem um melhor desfecho cirúrgico (p=0,052).
CONCLUSÕES: O número total de crises registrado não afeta o desfecho cirúrgico, que possivelmente é influenciado por múltiplos fatores. Pacientes com mais PLSz têm maior possibilidade de pior resultado cirúrgico.
Palavras-Chave: epilepsia, lobo temporal, cirurgia de epilepsia, eletroencefalografia.
Anterior temporal lobectomy is the current standard of care for patients with medically refractory medial temporal lobe epilepsy (MTLE)1,2. The goal of surgery is permanent disruption of the epileptogenic network and resultant seizure freedom3. Unfortunately, only 56-80% of patients who undergo temporal lobectomy1,2,4-9 remain seizure free. Prior to surgery, the location of ictal onset is most commonly defined by the recording of seizures during inpatient video-electroencephalography (VEEG) studies.
Despite the frequent use of ictal VEEG patterns for surgical planning, there is no consensus regarding the minimum quantity or quality of seizures that must be recorded for optimal patient selection for surgery. Ideally, ictal VEEG data should satisfy two criteria. First, the location and extent of the ictal onset zone is sufficiently characterized to provide a surgical target that will result in permanent disruption of the seizure network3,10. Secondly, the number and quality of recorded seizures is adequate to identify or exclude the possibility of a diffusely distributed or distinct second onset zone.
The theoretical minimal number of seizures needed to make accurate surgical decisions has been discussed in the literature. Van Ness et al.11 estimated that 17 seizures from the same EEG focus were required to conclude that at least 80% of a patient's seizures came from that source. A single seizure with a discordant EEG pattern increased the number required to 25. Blum et al.12 applied Bayes' theorem to seizure data from patients with proven bilateral temporal lobe epilepsy (TLE). They concluded that five consecutive concordant seizures ensures that 90% of the patient's seizures originate from that focus, but a single discordant seizure increases the required number of concordant seizures to 11. Of course, 90% from one focus does not guarantee postsurgical seizure freedom. Furthermore, recording 11-17 seizures is not always possible during inpatient VEEG, even if the duration of admission approaches 12 days12. This issue is further complicated by debate about seizure clustering. It has been suggested that clustered seizures are not truly independent seizures and have lower localization value than non-clustered seizures13.
Many practical factors affect the number of seizures recorded in the epilepsy monitoring unit (EMU). These include patient-level features, such as intrinsic seizure frequency. Clinical decisions also play a role, such as adjusting the duration of EMU stay based on early ictal EEG findings. Finally, social factors, as insurance coverage, bed availability and cost of admission, may have impact.
The impact of number of seizures recorded on outcome has not been confirmed. Further, the roles of the varied factors that determine how many seizures are recorded remain undefined. To our knowledge, there have not been studies on presurgical MTLE addressing these questions. We performed a retrospective cohort study to investigate the relationship between number of recorded seizures and surgical outcome. We hypothesized that seizure, patient and physician specific factors may influence the number of recorded seizures, and that accounting for these may enhance the application of VEEG results to surgical selection.
METHODS
Patient selection
We studied consecutive patients who had standard anterior temporal lobectomy at the Medical University of South Carolina (MUSC) for rMTLE14 between June 2006 and April 2010. We included patients with both presurgical VEEG at MUSC and at least one year of postoperative follow-up. This study was approved by the internal review board at MUSC.
Clinical features
For each subject, we recorded: (1) subject characteristics - onset and duration of epilepsy, handedness, gender, reported number of seizure semiologies, reported seizure frequency, number of antiepileptic drugs (AEDs) tried, side of resection, postsurgical seizure outcome; (2) diagnostic test results - routine EEG, EMU VEEG, intracranial EEG, MRI brain (1.5 & 3T), subtraction SPECT, interictal FDG-PET, histopathology and WADA test; and (3) presurgical VEEG characteristics - EMU duration (in days), number and types of seizures recorded and their EEG localization, number of days in EMU on which a seizure occurred (EMU seizure days) and attending physician of record. Ictal EEG patterns were categorized as concordant or discordant to the temporal lobe of resection, or poorly localized if onset location was unclear. Imaging studies were considered concordant or discordant to resection site. For each subject, discordant imaging was cataloged for individual imaging tests, and as a grouped variable, "any discordant imaging".
The duration in EMU was defined as total number of days spent in the EMU. Seizure days were defined as 24-hour periods in which subjects had at least one seizure. Seizure clusters were evaluated using two distinct definitions based on prior literature: >3 seizures in 24 hours15-17 and 2 seizures in <8 hours13. Seizure count adjusted for clusters was defined as the sum of the number of seizures not in a cluster plus the number of seizure clusters (i.e., a seizure cluster counted as a single seizure). Timing of first poorly localized seizure was quantified by calculating the ratio of the sequence number of the first poorly localized seizure to total seizure count.
Statistical analysis
The primary outcome of interest was seizure freedom at one year. We ran univariate analyses to test for associations with seizure freedom using χ2 or Fisher's exact test for categorical variables and t-tests for continuous variables. Explanatory variables with a p-value less than 0.25 in univariate tests were considered candidates for multivariable logistic regression modeling18 of the probability of a subject being seizure free at one year. All p-values reported in the multivariable logistic model were two-sided, with the type I error rate set at 0.05.
To explore factors that may confound the effect of seizure count on surgical outcome, we ran secondary analyses with the dependent variables: (a) number of seizures recorded in EMU (seizure count), (b) number of seizures that were poorly localized on VEEG, (c) number of days in EMU, (d) having intracranial EEG and (e) presence of seizure clusters. We used generalized linear models (GLM) to explore associations of independent variables with these dependent variables using a log link function for (a), (b), and (c), and a logit link for (d) and (e). For variables (a) and (c), Poisson (P) and Negative Binomial (NB) distributions were considered, and the most appropriate model was selected based on the likelihood ratio test. Because many subjects had zero poorly localized seizures, P, NB, zero-inflated P and zero-inflated NB distributions were considered for variable (b), and Vuong's statistic was used to select the most appropriate model19.
RESULTS
Subject characteristics
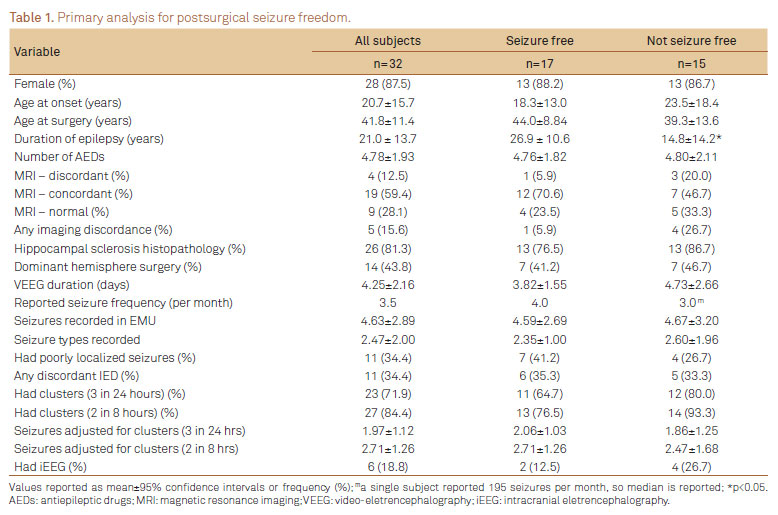
We identified 32 patients with presurgical VEEG admission at MUSC and at least one year of postoperative follow-up after anterior temporal lobectomy for treatment of rMTLE. Of these, 87.5% (28/32) were female and 84% (27/32) were right handed. Average age of epilepsy onset was 20.7 years and average age at surgery was 41.8 years (range 19-68), with mean duration of epilepsy of 21.4 years (range 2-46.5). Dominant temporal lobe resection occurred in 44% (14/32). At EMU admission, subjects reported median seizure frequency of 3.5 seizures per month (range 0.75-195), with an average of 1.8 clinical seizure semiologies (range 1-3). Subjects had taken an average of 4.8 different seizure medications (range 2-9) since onset of epilepsy. Six patients (17%) underwent subsequent intracranial EEG to improve localization of SOZ. At one year, 53% were seizure free (17/32), with no difference between dominant and non-dominant resection (p=0.76).
Diagnostic tests
During inpatient VEEG, scalp EEG revealed interictal epileptiform discharges (IED) concordant to resection in 63% (20/32), while 28% (9/32) had discordant IED. All subjects had brain MRI, with 72% (23/32) 3 T MRI (28% (9/32) were normal). Unilateral mesial temporal sclerosis or associated focal hippocampal abnormalities were found on brain MRI in 53% (17/32), and histopathology confirmed hippocampal sclerosis in 81% (26/32). Concordance between MRI abnormality and resection occurred in 59% (19/32) while 13% (4/32) had discordant MRI-EEG findings. Thirty-eight percent of subjects had SPECT scans (12/32), of which 67% (8/12) were concordant with resection. Presurgical PET was performed in 38% (12/32) of subjects, with 50% (6/12) concordant to resection. All subjects underwent WADA testing for lateralization of language (dominant hemisphere) and verbal memory.
VEEG characteristics
Average duration in EMU was 4.3 days (range 1-12) during which a mean of 4.6 seizures were recorded. Average number of seizures concordant to resection was 3.4 per subject. Two subjects had at least one ictal EEG pattern discordant to side of resection. Poorly localized seizures were observed in 11/32 patients, among whom there were an average of 3.1 poorly localized seizures per subject (range 1-7). The first poorly localized seizure occurred within 2 days of admission in 7/11 patients. Average number of seizure days was 2.0 per subject. Seizure clusters (>3 sz/ 24 hrs) occurred in 72% (23/32), and average seizure count adjusted for clusters was 2.0 per subject. Considering an alternative definition for seizure cluster (2 sz <8 hours), 85% (27/32) had clusters, and average seizure count adjusted for clusters was 2.6.
STATISTICAL ANALYSIS
Surgical outcome as dependent variable
At one-year postsurgery, Engel Class 1 was achieved in 63% of subjects (20/32), with Class 1A in 53% (17/32). Mean number of seizures recorded were 4.6 for seizure free subjects and 4.7 for those not seizure free (p=0.941). Surprisingly, longer duration of epilepsy at surgery was associated with postoperative seizure freedom (p=0.013). Four of 11 subjects with poorly localized seizures during VEEG became seizure free after surgery (p=0.472). Number of poorly localized seizures was not significantly associated with seizure freedom (p=0.712). However, when duration of epilepsy was controlled in the regression model, there was a trend for more poorly localized seizures recorded to be associated with recurrent postoperative seizures (p=0.052). Surgical outcome was not correlated with having had iEEG (p=0.394) or histopathology (p=0.659) (Table 1).
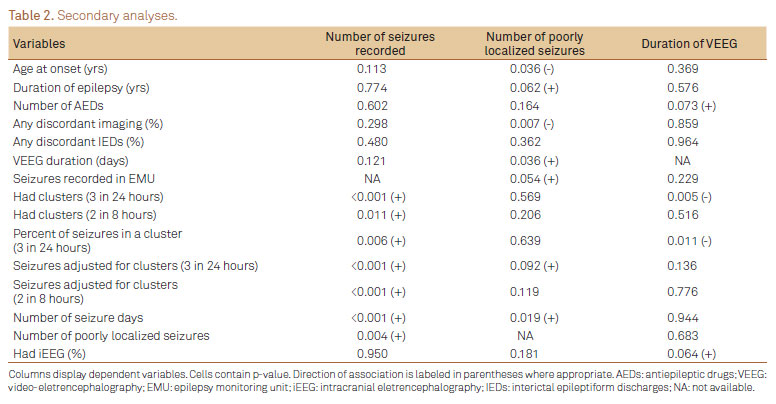
Number of seizures captured as dependent variable
As expected, patients with seizure clusters and more EMU seizure days showed a trend towards higher seizure count during presurgical VEEG (p<0.01 for both). Older age at admission was associated with fewer seizures captured (p=0.002). Interestingly, subject-reported seizure frequency and reported number of seizure semiologies did not predict seizures recorded (p=0.225 and 0.676 respectively). Having more poorly localized seizures (p=0.003) was associated with more total seizures recorded. We, therefore, evaluated the timing of the first poorly localized seizure relative to the total number of seizures captured for each subject. Ratio of sequence number of first poorly localized seizure to total seizure count ranged from 0.12-1.0, suggesting that earlier occurrence was not associated with higher seizure count overall or prolongation of VEEG duration (p=0.086 and p=0.453 respectively) (Table 2).
Number of poorly localized seizures recorded as dependent variable
The presence of poorly localized seizures recorded was associated with more seizures recorded by VEEG, and showed a trend towards worse postsurgical outcome when duration of epilepsy was controlled (p=0.053). Factors associated with the number of poorly localized seizures recorded during VEEG monitoring were therefore identified. These included: (a) younger age at epilepsy onset (p=0.036), (b) longer duration of EMU admission (p=0.036), (c) more EMU seizure days (p=0.019), and (d) any discordant imaging (p=0.007).
Intracranial EEG as dependent variable
Although having intracranial EEG (iEEG) did not correlate with surgical outcome, we hypothesized that subjects having iEEG prior to surgery may have been selected based on more poorly localized seizures and more total seizures recorded (to ensure adequate sampling). Six of 32 subjects (19%) had iEEG after scalp VEEG. Having had iEEG was not associated with having poorly localized seizures on VEEG, discordant imaging, discordant ictal or interictal EEG findings, higher number of EMU seizure days or higher total seizure count (p>0.05 for all). However, there was a trend towards higher number of AEDs used by patients who underwent iEEG (mean 6.2 versus 4.4, p=0.051).
Duration of VEEG monitoring as dependent variable
There are many factors that may affect seizure count which are beyond the control of the epileptologist. Duration of EMU admission, however, may be determined directly by the attending physician. Thus, the impact of multiple variables on VEEG duration was explored. Having >3 seizures in 24 hours was associated with shorter durations (p=0.005). Nevertheless, number of poorly localized seizures, having discordant interictal EEG or imaging findings, duration of epilepsy, subject-reported seizure frequency and number of seizure semiologies were not correlated with the number of days of VEEG (p>0.05 for all). Furthermore, the day on which first poorly-localized seizure occurred (range 1-5) was not associated with subsequent or total number of VEEG days.
DISCUSSION
Ictal EEG is the most commonly used method to determine the ictal onset zone in patients with medically refractory epilepsy. It has also been employed as a predictive variable regarding postoperative seizure control5,9,20-22. Interestingly, focal MRI lesions may be more predictive of postoperative seizure remission6,8,23,24. This discrepancy may be the result of inadequate seizure sampling. Prior reports have suggested that the theoretical minimum seizure count necessary to rule out a potential second seizure focus is higher than the number of seizures typically recorded for surgical decision making. We present, what is to our knowledge, the first exploration of factors contributing to the number of seizures recorded during EMU admissions and the subsequent association with outcome after temporal lobectomy.
We report three important observations: first, the number of seizures recorded pre surgically did not correlate to postsurgical seizure freedom (4.6 versus 4.7). Second, this similarity may be due to the variety of factors that affect the ability to record seizures in the EMU, many of which are beyond the control of the epileptologist. Third, we observed a trend towards recurrent seizures after surgery in patients with a higher number of poorly localized seizures.
Our sample size, while typical for a three-year period at a comprehensive epilepsy center, nonetheless, restricted our ability to detect potentially significant factors and perform subgroup analyses. This factor may explain the correlation between longer duration of epilepsy and better surgical outcome, which is contrary to other reports4,25-27. Notwithstanding sample size, we conclude that the lack of difference in seizure count among outcome groups was a true finding rather than a result of underpowering.
Commonly used methods to increase seizure capture during VEEG include medication withdrawal and sleep deprivation. Active seizure induction has been used historically28-30, but it may sometimes lead to false localization of onset. The simplest approach, prolongation of VEEG admission, is often limited by social factors, such as patient/family employment, or cost. We were surprised that EMU duration showed no correlation with number or timing of poorly localized seizures, discordant interictal EEG or discordant imaging. This suggests that the association between longer EMU duration and identification of poorly localized seizures was not driven by epileptologist prolongation of the EMU stay.
That higher numbers of poorly localized seizures may be associated with seizure recurrence is intuitive and suggests higher likelihood of an obscure seizure source, diffuse epileptogenic network or multiple seizure foci. Association of more poorly localized seizures with younger age of onset and presence of discordant imaging further support this conclusion. Importantly, our data demonstrated that the more seizures are recorded, the more likely a poorly localized seizure will be recorded. Furthermore, after controlling for duration of epilepsy, there was a trend towards recurrent postsurgical seizures if even one poorly localized seizure was recorded. Overall, these data highlight a potential link between seizure count and detection of poorly localized seizures, which may impact surgical outcome.
Finally, the lack of association between number of seizures recorded and surgical outcome is probably not due to lack of true correlation. We hypothesize that an appropriate target number exists for improvement of surgical outcome and varies based on subject specific factors. Future elucidation of these factors may enhance the predictive role of ictal EEG as part of a comprehensive presurgical evaluation.
In conclusion, we did not find a direct relationship between the number of seizures recorded presurgically and surgical outcome. Further work is necessary to clarify the role of specific features, such as poorly localized seizures, on the VEEG acquisition process and subsequent surgical outcome. Improved understanding of the importance of seizure count for individualized surgical decision-making may augment the role of ictal EEG in postoperative outcome prediction.
Received 06 April 2012
Received in final form 21 May 2012
Accepted 28 May 2012
Support: South Carolina Clinical & Translational Research Institute, Medical University of South Carolina's CTSA, National Institutes of Health/National Center for Research Resources (NIH/NCRR) Grant Number UL1RR029882. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR.
Conflict of interest: There is no conflict of interest to declare.
- 1. Wiebe S, Blume WT, Irvin JP, Liasziw ME. A randomized controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311-318.
- 2. Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 2003;44:741-751.
- 3. Engel J Jr, International League Against Epilepsy (ILAE). A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001;42:796-803.
- 4. Cohen-Gadol AA, Wilhelmi BG, Collingnon F, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. J Neurosurg 2006;104:513-524.
- 5. Bell ML, Rao S, So EL, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia 2009;50:2053-2060.
- 6. Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128:1188-1198.
- 7. Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology 2005;65:912-918.
- 8. Weishmann UC, Larkin D, Varma T, Eldridge P. Predictors of outcome after temporal lobectomy for refractory temporal lobe epilepsy. Acta Neurol Scand 2008;118:306-312.
- 9. Tatum WO 4th, Benbadis SR, Hussain A, et al. Ictal EEG remains the prominent predictor of seizure-free outcome after temporal lobectomy in patients with normal brain MRI. Seizure 2008;17;631-636.
- 10. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 2002;43;219-227.
- 11. Van Ness PC, So NK, Collura T, Beck GJ, Luders H. Ictal and interictal EEG: what constitutes an adequate sample for epilepsy surgery? Epilepsia 1994;31:623.
- 12. Blum D. Prevalence of bilateral partial seizure foci and implications for electroencephalographic telemetry monitoring and epilepsy surgery. Electroencephalogr Clin Neurophysiol 1994;19:329-336.
- 13. Todorov AB, Lesser RP, Uematsu SS, Yankov YA, Todorov AA Jr. Distribution in time of seizures during presurgical VEEG monitoring. Neurology 1994;44:1060-1064.
- 14. Haut S, Legatt A, O'Dell C, Moshé SL, Shinnar S. Seizure lateralization during EEG monitoring in patients with bilateral foci: the cluster effect. Epilepsia 1997;38:937-940.
- 15. Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology 2005;65:1313-1315.
- 16. Rose AB, McCabe PH, Gilliam FG, et al. Occurrence of seizure cluster and status epilepticus during inpatient video-EEG monitoring. Neurology 2003;60:975-978.
- 17. Haut SR, Shinnar S, Moshé SL. Seizure clustering: risk and outcomes. Epilepsia 2005;46:146-149.
- 18. Mickey J, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989;129:125-137.
- 19. Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 1989;57:307-333.
- 20. Schulz R, Lüders HO, Hoppe M, Tuxhorn I, May TT, Ebner A. Interictal EEG and ictal scalp EEG propagation are highly predictive of surgical outcome in mesial temporal lobe epilepsy. Epilepsia 2000;41:564-570.
- 21. Kelemen A, Barsi P, Eross L, et al. Long-term outcome after temporal lobe surgery-prediction of late worsening of seizure control. Seizure 2006;15:49-55.
- 22. Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure 2000;9:407-411.
- 23. Elsharkawy AE, Alabbasi AH, Pannek H, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. J Neurosurg 2009;110:1135-1146.
- 24. Berkovic SF, McIntosh AM, Kalnins RM, et al. Preoperative MRI predicts outcome of temporal lobectomy. Neurology 1995;45:1358-1363.
- 25. Eliashiv SD, Dewar S, Wainwright I, Engel J Jr, Fried I. Long-term follow-up after temporal lobe resection for lesions associated with chronic seizures. Neurology 1997;48:621-626.
- 26. Sirven JI, Malamut BL, O'Connor MJ, Sperling MR. Temporal lobectomy outcome in older versus younger adults. Neurology 2000;54:2166-2170.
- 27. Lowe NM, Eldridge P, Varma T, Wieshmann UC. The duration of temporal lobe epilepsy and seizure outcome after epilepsy surgery. Seizure 2010;19:261-263.
- 28. Guaranha MSB, Garzon E, Buchpiguel CA, Tazima SE, Yacubian EMT, Sakamoto AC. Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia 2005;46:69-75.
- 29. Lennox W, Gibbs F, Gibbs E. Effect on the encephalogram of drugs and conditions which influence seizures. Arch Neurol Psychiat 1936;36:1236-1245.
- 30. Jonas J, Vignal JP, Baumann C, et al. Effect of hyperventilation on seizure activation: potentiation by withdrawal of antiepileptic drug. J Neurol Neurosurg Psychiatry 2011;82;928-930.
Publication Dates
-
Publication in this collection
12 Sept 2012 -
Date of issue
Sept 2012
History
-
Received
06 Apr 2012 -
Accepted
28 May 2012 -
Reviewed
21 May 2012