Abstract
Introduction:
Kidney Donor Profile Index (KDPI) has been incorporated in the United States to improve the kidney transplant allocation system.
Objectives:
To evaluate deceased kidney donors’ profile using KDPI and compare to the previous United Network for Organ Sharing (UNOS) definition of expanded criteria donors (ECD) and assess the KDPI applicability to predict five-year graft survival and renal function in our sample.
Methods:
Retrospective cohort of 589 kidney transplants from deceased donors performed from January 2009 to May 2013 with follow-up until May 2018.
Results:
In 589 kidney transplants, 36.6% of donors were classified as ECD and 28.8% had KDPI ≥ 85%. Mean KDPI was 63.1 (95%CI: 60.8-65.3). There was an overlap of standard and ECD in KDPI between 60 and 95 and a significantly lower death-censored graft survival in KDPI ≥ 85% (78.6%); KDPI 0-20: 89.8%, KDPI 21-59: 91.6%, and KDPI 60-84: 83.0%; p = 0.006. The AUC-ROC was 0.577 (95%CI: 0.514-0.641; p = 0.027). Renal function at 5 years was significantly lower according to the incremental KDPI (p < 0.002). KDPI (HR 1.011; 95%CI 1.001-1.020; p = 0.008), donor-specific antibodies (HR 2.77; 95%CI 1.69-4.54; p < 0.001), acute rejection episode (HR 1.73; 95%CI 1.04-2.86; p = 0.034) were independent and significant risk factors for death-censored graft loss at 5 years.
Conclusion:
In our study, 36.6% were classified as ECD and 28.8% had KDPI ≥ 85%. KDPI score showed a moderate power to predict graft survival at 5 years. Renal function was significantly lower in patients with higher KDPI.
Keywords:
Kidney Transplantation; Donor Selection; Graft Survival
Resumo
Introdução:
O Índice de Perfil de Doadores de Rins (KDPI) foi adotado nos Estados Unidos para melhorar o sistema de alocação de transplantes renais.
Objetivos:
avaliar o perfil dos doadores de rim falecidos usando o KDPI e comparar com a definição anterior do United Network for Organ Sharing (UNOS) de doadores de critérios expandidos (DCE) e avaliar a aplicabilidade do KDPI para prever a sobrevida do enxerto em cinco anos e a função renal em nossa amostra.
Métodos:
Coorte retrospectiva de 589 transplantes renais de doadores falecidos, realizada de janeiro de 2009 a maio de 2013, com acompanhamento até maio de 2018.
Resultados:
Em 589 transplantes renais, 36,6% dos doadores foram classificados como DCE e 28,8% apresentaram KDPI ≥ 85%. O KDPI médio foi de 63,1 (IC 95%: 60,8-65,3). Houve uma sobreposição de padrão e DCE no KDPI entre 60 e 95 e uma sobrevida do enxerto censurada por óbito significativamente menor no KDPI ≥ 85% (78,6%); KDPI 0-20: 89,8%, KDPI 21-59: 91,6% e KDPI 60-84: 83,0%; p = 0,006. A ASC-ROC foi de 0,577 (IC 95%: 0,514-0,641; p = 0,027). A função renal aos 5 anos foi significativamente menor de acordo com o aumento do KDPI (p <0,002). KDPI (HR 1.011; 95% CI 1.001-1.020; p = 0.008), anticorpos específicos contra doadores (HR 2,77; 95% CI 1,69-4,54; p <0,001), episódio de rejeição aguda (HR 1,73; 95% CI 1,04-2,86; p = 0,034) foram fatores de risco independentes e significativos para perda do enxerto censurada por óbito em 5 anos.
Conclusão:
Em nosso estudo, 36,6% foram classificados como DCE e 28,8% apresentaram KDPI ≥ 85%. O escore KDPI mostrou potencial moderado para prever a sobrevida do enxerto em 5 anos. A função renal foi significativamente menor nos pacientes com maior KDPI.
Palavras-chave:
Transplante de Rim; Seleção de Doadores; Sobrevivência de Enxerto
Introduction
Expanded criteria donor (ECD) has been used in many medical centers, confronting the dilemma of accepting organs with expected lower allograft survival or discarding the organs and maintaining the patient on dialysis with a considerable mortality risk while waiting for another standard donor offer11 McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991-2001. Nephrol Dial Transplant. 2002 Dec;17(12):2212-9.
2 Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001 Mar;12(3):589-97.-33 Pérez-Sáez M, Arcos E, Comas J, Crespo M, Lloveras J, Pascual J, et al. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis. Am J Transplant. 2016;16(9):2724-33.. The 2001 United Network for Organ Sharing (UNOS) definition of ECD was developed as a binary variable to assess the risk of graft loss, however not all ECDs have the same risk. Trying to improve the prediction of ECD outcomes, an index was developed, namely the Kidney Donor Profile Index (KDPI), which is a numerical measure that combines 10 donor factors. This index is described as a percentile measure of which higher values are associated to worse outcomes44 Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009 Jul;88(2):231-6.,55 Organ Procurement and Transplantation Network (OPTN). US Department of Health and Human Services. Data Reports - Regional Data [Internet]. USA: HHS; 2014; [cited 2016 May 4]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/
https://optn.transplant.hrsa.gov/data/vi...
. Since 2014, KDPI has been used in the US kidney allocation system (KAS). The primary purpose of KDPI is the implementation of the “longevity matching” concept into the KAS. Candidates with longer estimated post-transplant longevity (EPTS score of 20% or less) will receive priority for kidneys from donors with a KDPI of 20%; on the other hand, donors with a KDPI ≥ 85% are thought to be equivalent to an ECD donor and are considered as a high-risk kidney55 Organ Procurement and Transplantation Network (OPTN). US Department of Health and Human Services. Data Reports - Regional Data [Internet]. USA: HHS; 2014; [cited 2016 May 4]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/
https://optn.transplant.hrsa.gov/data/vi...
. In Brazil, an equation that quantifies the risk based on our donor profile does not exist. The aim of our study was to evaluate the profile of deceased kidney donors by using the KDPI calculator compared to the previous UNOS definition of ECD and the applicability to predict a five-year renal function and graft survival in our sample.
Patients And Methods
This was a retrospective cohort study. The eligible population was all sequentially deceased donor kidney transplant recipients transplanted from January 2009 to May 2013 with a follow up until May 2018. Inclusion criteria were adult kidney recipients with at least 3 months of follow-up. We excluded kidney transplants (KTs) combined with another organ and all the cases with missing data necessary to calculate the KDPI scores. Clinical data from our local transplant database and medical records including baseline demographic characteristics from donors and recipients, transplant characteristics and clinical follow-up, return to dialysis, and death were collected. This study was undertaken following the principles stated by the World Medical Association Declaration of Helsinki and was submitted and approved by the Institutional Ethics Review Board.
Our kidney allocation system followed the human leukocyte antigen (HLA) compatibility score as a major weight variable. The crossmatch was performed for all transplants by cytotoxicity and flow cytometry. Our acceptance criteria of older donors followed the UNOS ECD definition associated with the pre-implant biopsy results. The biopsy criteria included the ECD donor, acute renal injury and macroscopy abnormalities.
For analyses purpose, the donors were classified by the previous UNOS criteria66 Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002 Nov;74(9):1281-6. and KDPI using the formula available on the website of the OPTN (https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator)55 Organ Procurement and Transplantation Network (OPTN). US Department of Health and Human Services. Data Reports - Regional Data [Internet]. USA: HHS; 2014; [cited 2016 May 4]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/
https://optn.transplant.hrsa.gov/data/vi...
. KDPI was grouped based on its range for comparison, i.e., 0-20% for the 20% of the best donors and ≥ 85% for donors equivalent to ECDs in the US KAS. The intermediate range between 21-59% corresponded to the standard criteria donors (SCDs) while 60-84% range included the overlapped ECDs and SCDs.
Our sample did not involve any donor with cardiac death because Brazilian law does not allow it. The delayed graft function was defined as the necessity of at least one dialysis session within the first week after kidney transplantation. Donor specific antibodies (DSA) were considered for mean fluorescence intensity above 1000 in the pre-transplant sera screening in all recipients. Definition of acute rejection episode was biopsy-proved. The immunosuppressive regimen does not differ between SCD and ECD (anti-CD 25, tacrolimus, mycophenolic acid, and prednisone). Induction therapy with antithymocyte globulin was used in presence of DSA, panel reactive antibody above 50%, and cold ischemia time > 24 hours. The graft failure was defined as return to dialysis, preemptive re-transplantation, or death with functioning graft. The graft survival, death-censored graft survival, and glomerular filtration rate (GFR) after one, three, and five years post-transplantation were the evaluated outcomes. GFR was estimated by the chronic kidney disease epidemiology (CKD-EPI) formula77 Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150(9):604-12. and compared among the KDPI score ranges.
Statistical analysis
Qualitative variables are presented as frequency and percentage. Quantitative variables with a normal distribution are reported as mean and standard deviation (SD) or 95% confidence interval (95%CI). The differences between mean scores were analyzed by the student t-test or ANOVA. Actuarial graft survivals were performed by the Kaplan-Meier and log-rank methods. The assessment of risk for graft loss was performed by the Cox uni and multivariate analyses and shown as hazard ratios (HRs) with 95%CI. In the multivariate analysis we included only the variables with a p value < 0.05 in the univariate analysis. We also performed the receiver operational characteristic (ROC) curves for assessing the predictive ability of KDPI to estimate the death-censored graft-failure. The value defined as cut-off was determined by the maximum of Youden index (J = sensitivity + specificity − 1). A p value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS V 23.088 International Business Machines (IBM). IBM SPSS statistics for Windows. Version 21. Armonk, NY: IBM Corp.; 2013..
Results
During the study period, 744 deceased donor kidney transplants were performed of which 589 transplants fulfilled the inclusion criteria. Recipients aged less than 18 years (n = 126) and the kidney transplants combined with other solid organs (n = 29) were excluded. The demographic data are shown in Table 1.
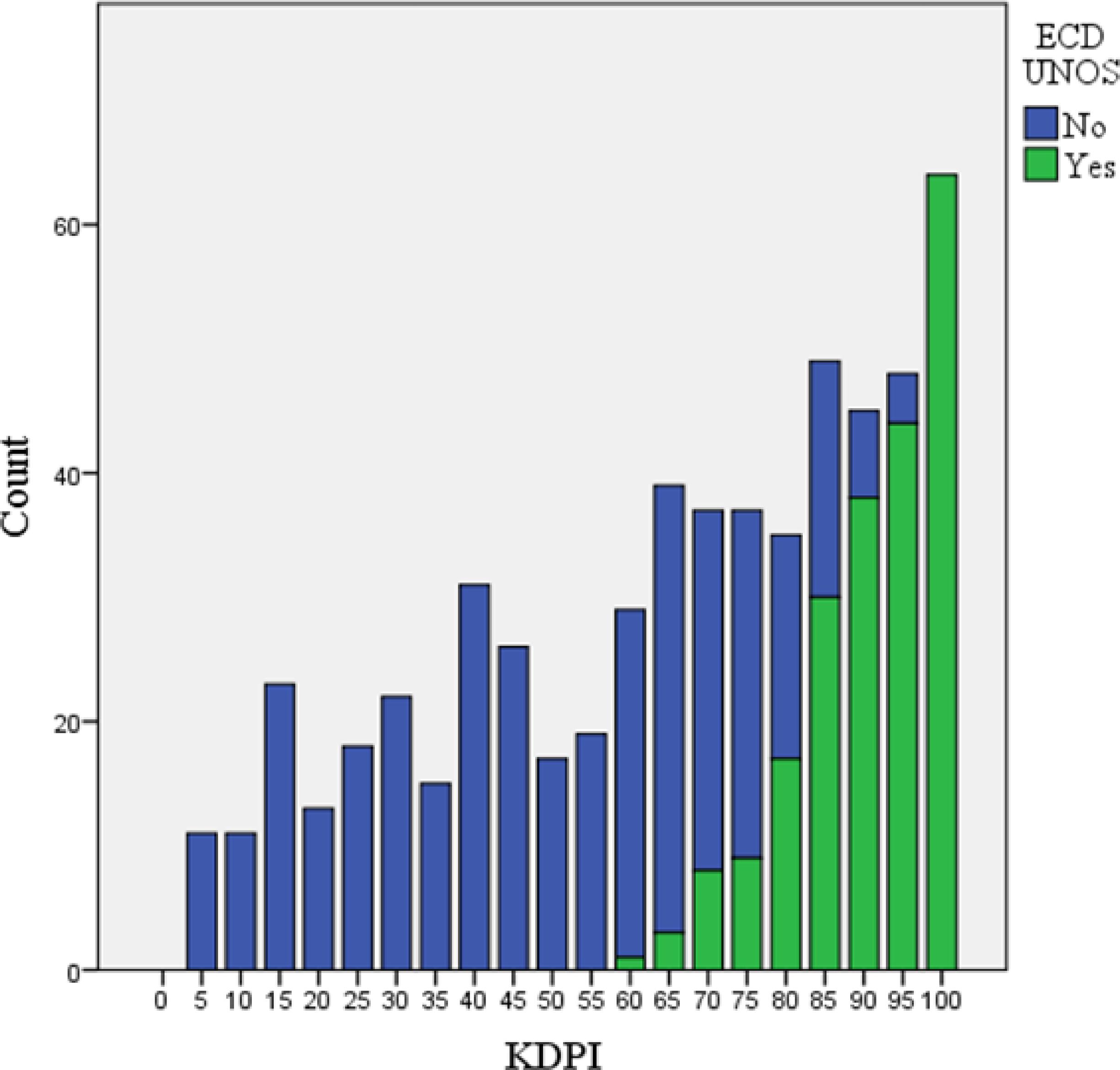
Kidney transplants from ECDs by the previous UNOS criteria were 36.3%, and 28.8% had KDPI ≥ 85%. The mean KDPI was 63.1 (95%CI: 60.8-65.3). When comparing donors according to the KDPI and UNOS criteria, all KDPIs inferior to 60 were considered as standard criteria donor (SCDs) and all KDPI that equaled or exceeded 95% were regarded as ECDs. There was an overlap of SCDs and ECDs in KDPI between 60 and 95% (Figure 1).
The mean recipient age (years) in the KDPI categories was 0-20: 44.79 (95%CI: 41.1-48.4); 21-59: 45.45 (95%CI: 43.6-47.2); 60-84: 49.96 (95%CI: 48.2-51.7) and ≥ 85: 55.2 (95%CI: 53.6-56.8). There was a significant difference in recipient age between KDPI ≥ 60 and ≥ 85 when compared to KDPI < 60 (p = 0.002 and p < 0.001, respectively). There was no significant difference when comparing recipient age with KDPI between 0-20 and 21-59%.
The global graft survival according to KDPI is presented in Figure 2. There was a significantly lower 5-year graft survival for KDPI ≥ 85% (59.6%) when compared to the other ranges (KDPI 0-20: 80.1%; KDPI 21-59: 79.9% and KDPI 60-84: 73.9%; p < 0.001). There were 82 deaths in the study period. The main causes were infection (n = 49), cardiovascular (n = 13), and neoplasm (n = 7). The causes of return to dialysis (n = 70) were immunological (n = 36), infection (n = 18), surgical (n = 3), recurrence of original disease (n = 2), and others (n = 11). Nine grafts had primary nonfunctioning.
There was also a significantly lower 5-year death-censored graft survival in KDPI ≥ 85 (78.6%); KDPI 0-20: 89.8%, KDPI 21-59: 91.6%, and KDPI 60-84: 83.0%; p = 0.006 (Figure 3).
The AUC-ROC for graft loss was 0.577 (95%CI: 0.514-0.641; p = 0.027). KDPI of 71% presented the best sensitivity (55.7%) and specificity (55.7%). The KDPI of 85% showed 39% sensibility and 73% specificity for 5-year death-censored graft loss.
Kidney function at 1 and 5 years of follow-up was significantly lower with higher KDPI (p < 0.002) (Figure 4).
The univariate analysis for the risk of death-censored graft loss is presented in Table 2. For each KDPI incremental point there was a 1.1% (HR 1.01; 95%CI 1.003-1.020 p = 0.011) increased risk of 5-year graft loss. Kidneys from ECDs defined by the UNOS criteria presented 207% increased risk for graft loss when compared to SCDs (p = 0.001). The presence of DSA also showed an increased risk (HR 2.48; 95%CI 1.54-3.99; p < 0,001). Among 98 recipients with DSA, 36 (36.7%) died or returned to dialysis in 5 years after transplantation when compared to 123 (25.3%) among 487 without DSA. The pre-transplantation T cell flow cytometry crossmatch was negative in all recipients; however, 21 had positive B cell flow crossmatch. The later was a risk factor for graft loss (HR 2.64; 95%CI 1.13-6.17; p = 0.002). The PRA class II above 80% increased the risk of graft loss (HR 3.24; 95%CI 1.41-7.41; p = 0.005). For each additional hour of cold ischemia time, the risk of graft loss was increased by 6% (CIT measured in minutes: HR 1.001; 95%CI 1.00-1.001; p = 0.032.). The recipient age and delayed graft function were not significant risk factors for graft loss.
The multivariate analysis is also shown in Table 2. The PRA Class II and CIT lost significance (p = 0.350 and 0.161, respectively). KDPI (HR 1.00; 95%CI 1.00-1.02; p = 0.008), DSA (HR 2.77; 95%CI 1.69-4.54; p < 0.001), and acute rejection episode (HR 1.73; 95%CI 1.04-2.86; p = 0.034) remained as an independent and significant risk factors for death-censored graft loss at 5 years.
The previous UNOS criteria were not included in the multivariate analysis because they are strongly correlated with KDPI; thus, the 4 variables in the UNOS criteria for EDC were included in KDPI.
Discussion
In our cohort, 36.3% of kidney transplants were from ECDs matching the UNOS criteria and 28.8% had KDPI ≥85%. In comparison, an US study found 17.3% ECD and 9.7% KDPI > 85%99 Rege A, Irish B, Castleberry A, Vikraman D, Sanoff S, Ravindra K, et al. Trends in usage and outcomes for expanded criteria donor kidney transplantation in the United States characterized by kidney donor profile index. Cureus. 2016 Nov;8(11):e887. while a Spanish study showed 41.9% ECD and 35% KDPI ≥ 85%1010 Arias-Cabrales C, Pérez-Sáez MJ, Redondo-Pachón D, Buxeda A, Burballa C, Bermejo S, et al. Usefulness of the KDPI in Spain: a comparison with donor age and definition of standard/expanded criteria donor. Nefrologia. 2018 Sep/Oct;38(5):503-13.. A South Brazilian study of 346 renal transplants found a 30.6% ECD1111 Helfer MS, Vicari AR, Spuldaro F, Gonçalves LF, Manfro RC. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation in a Brazilian center. Transplant Proc. 2014 Jul/Aug;46(6):1727-9.. Studies from other Brazilian regions presented 9.4% (Ceará) and 16.5% (São Paulo) of transplants using ECD1212 Sandes-Freitas TV, Felipe CR, Aguiar WF, Cristelli MP, Tedesco-Silva H, Medina-Pestana JO. Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals. PLoS One. 2015 Dec;10(12):e0144188.,1313 Mota LS, Oliveira CMS, Pinheiro Junior FML, Santos LCO, Nóbrega DG, et al. Comparative study between kidney transplantation with expanded criteria deceased donor and standard criteria donor in a single center in Brazil. J Bras Nefrol. 2016;38:348-57.. In our sample, 45% of renal transplants were allocated from other states of Brazil. This means a higher ECD acceptability of our state when compared to other states in Brazil, which is similar to the Spanish model1010 Arias-Cabrales C, Pérez-Sáez MJ, Redondo-Pachón D, Buxeda A, Burballa C, Bermejo S, et al. Usefulness of the KDPI in Spain: a comparison with donor age and definition of standard/expanded criteria donor. Nefrologia. 2018 Sep/Oct;38(5):503-13.,1212 Sandes-Freitas TV, Felipe CR, Aguiar WF, Cristelli MP, Tedesco-Silva H, Medina-Pestana JO. Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals. PLoS One. 2015 Dec;10(12):e0144188.,1313 Mota LS, Oliveira CMS, Pinheiro Junior FML, Santos LCO, Nóbrega DG, et al. Comparative study between kidney transplantation with expanded criteria deceased donor and standard criteria donor in a single center in Brazil. J Bras Nefrol. 2016;38:348-57..
Our study demonstrated a considerable overlap in the KDPI distribution between SCD and ECD categories also noticed by Rao et al. and Woodside et al. This overlap was in KDPI range between 60 and 95%44 Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009 Jul;88(2):231-6.,1414 Woodside KJ, Merion RM, Leichtman AB, Santos R, Arrington CJ, Rao PS, et al. Utilization of kidneys with similar kidney donor risk index values from standard versus expanded criteria donors. Am J Transplant. 2012 Jun;12(8):2106-14.. The KDPI represents a substantial improvement in scale and interpretability relative to the less accurate SCD versus ECD classification. It was developed to improve the organ allocation and decrease kidney discard. Our sample allografts from ECD had HR 2.07 5-year death-censored graft loss when compared to SCD. Molnar et al. and Reeves-Daniel showed HR of 1.82 and 1.45, respectively1515 Molnar MZ, Streja E, Kovesdy CP, Shah A, Huang E, Bunnapradist S, et al. Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes. Am J Kidney Dis. 2012 Jun;59(6):841-8.,1616 Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011 May;11(5):1025-30.. American studies found the relative risk of 1.7 to 1.77 for graft loss in ECD transplants1717 Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;(3 Suppl 4):114-25.
18 Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2(8):701-11.-1919 Sung RS, Guidinger MK, Leichtman AB, Lake C, Metzger RA, Port FK, et al. Impact of the expanded criteria donor allocation system on candidates for and recipients of expanded criteria donor kidneys. Transplantation. 2007 Nov;84(9):1138-44.. Mezrich et al. showed HR equal to 1.49 (95%CI 0.98-2.27) of graft failure and patient death for ECD kidney recipients (not significant for recipients between 40 and 59 years)2020 Mezrich JD, Pirsch JD, Fernandez LA, Foley DP, Bellingham JM, Odorico JS, et al. Differential outcomes of expanded-criteria donor renal allografts according to recipient age. Clin J Am Soc Nephrol. 2012 Jul;7(7):1163-71.. The justification for the use of ECD is the beneficial effect when compared to the patients remaining on the waiting list, especially those older than 40 years and with diabetes, who would most likely not survive long waiting periods33 Pérez-Sáez M, Arcos E, Comas J, Crespo M, Lloveras J, Pascual J, et al. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis. Am J Transplant. 2016;16(9):2724-33.,2121 Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008 Sep;52(3):553-86.
22 Port FK, Dykstra DM, Merion RM, Wolfe RA. Trends and results for organ donation and transplantation in the United States, 2004. Am J Transplant. 2005 Apr;5(4 Pt 2):843-9.-2323 Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093-109..
In our study, each KDPI increment of 1% was independently associated to 1% graft loss risk at 5 years in univariate as well as multivariate analyses. Arias-Cabrales et al. found 3% increase in the graft loss risk for each KDPI increment1010 Arias-Cabrales C, Pérez-Sáez MJ, Redondo-Pachón D, Buxeda A, Burballa C, Bermejo S, et al. Usefulness of the KDPI in Spain: a comparison with donor age and definition of standard/expanded criteria donor. Nefrologia. 2018 Sep/Oct;38(5):503-13.. Gandolfini et al. observed higher KDPI values associated to poorer graft outcomes in an Italian cohort of 442 marginal kidneys allocated as single or dual kidney transplantation2424 Gandolfini I, Buzio C, Zanelli P, Palmisano A, Cremaschi E, Vaglio A, et al. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014 Nov;14(11):2515-25.. Woodside et al. found a similar graft survival for ECD and SCD in each KDRI range1414 Woodside KJ, Merion RM, Leichtman AB, Santos R, Arrington CJ, Rao PS, et al. Utilization of kidneys with similar kidney donor risk index values from standard versus expanded criteria donors. Am J Transplant. 2012 Jun;12(8):2106-14.. Other authors also showed a relationship between higher KDPI, risk of graft failure, and recipient death2525 Han M, Jeong JC, Koo TY, Jeon HJ, Kwon HY, Kim YJ, et al. Kidney donor risk index is a good prognostic tool for graft outcomes in deceased donor kidney transplantation with short, cold ischemic time. Clin Transplant. 2014;28(3):337-44.
26 Lehner LJ, Kleinsteuber A, Halleck F, Khadzhynov D, Schrezenmeier E, Duerr M, et al. Assessment of the Kidney Donor Profile Index in a European cohort. Nephrol Dialys Transplant. 2018 Aug;33(8):1465-72.-2727 Snyder J, Salkowski N, Wey A, Israni AK, Schold JD, Segev DL, et al. Effects of high-risk kidneys on scientific registry of transplant recipients program quality reports. Am J Transplant. 2016 Sep;16(9):2646-53.. The KDRI has already been validated in the Dutch population2828 Peters-Sengers H, Heemskerk MBA, Geskus RB, Kers J, Van Der Heide JJH, Berger SP, et al. Validation of the prognostic Kidney Donor Risk Index scoring system of deceased donors for renal transplantation in the Netherlands. Transplantation. 2018 Jan;102(1):162-70.. In Spain, the KDPI and KDRI were validated for ECDs2929 Martín RMGM, Díaz JAR, Molina MC, Tornel BMC, Soto JB, Ortega AO, et al. Validation of KDRI/KDPI for the selection of expanded criteria kidney donors. Nefrologia. 2018 May/Jun;38(3):297-303..
Despite the acceptable graft survival results, the discard rates have increased in US, especially for high KDPI kidneys. From 2012 to 2014, 18.3% of available kidneys for transplant were discarded. The discard rates increased 50.6% for KDPIs > 80% and 71.6% for KDPI > 95%3030 Bae S, Massie AB, Luo X, Anjum S, Desai NM, Segev DL. Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI). Am J Transplant. 2016 Jul;16(7):2202-7.. In Europe, the discard rate was 14% in the same period3131 Eurotransplant International Foundation (EIF). Eurotransplant Annual Report 2014 [Internet]. Netherlands: EIF; 2014. Disponível em: https://www.eurotransplant.org/cms/mediaobject.php?file=ar_2014.pdf
https://www.eurotransplant.org/cms/media...
. This difference may be partially due to the “fear of flagging” worse graft and patient survival in the program-specific reports on kidney transplantation in US; these underperforming programs may have a penalty2727 Snyder J, Salkowski N, Wey A, Israni AK, Schold JD, Segev DL, et al. Effects of high-risk kidneys on scientific registry of transplant recipients program quality reports. Am J Transplant. 2016 Sep;16(9):2646-53.. In 2017, in Brazil, we estimated an overall kidney discard rate of 30% and there is no register for donor risk index3232 Registro Brasileiro de Transplantes (RBT). 2017 - Dimensionamento dos Transplantes no Brasil e em cada estado. São Paulo: ABTO; 2018; XXIII(4):1-104. Disponível em: http://www.abto.org.br/abtov03/Upload/file/RBT/2017/rbt-imprensa-leitura-compressed.pdf
http://www.abto.org.br/abtov03/Upload/fi...
.
Lehner et al. observed a GFR reduction as KDPI increased (65.8, 60.4, 46.1, and 35.2 mL/min/1.73 m2 for KDPI < 20, 21-34, 35-85, and > 85%, respectively). The reduction rate was similar in the different groups during the follow-up; however, they included a GFR of 0 mL/min/1.73 m2 for patients with graft loss2626 Lehner LJ, Kleinsteuber A, Halleck F, Khadzhynov D, Schrezenmeier E, Duerr M, et al. Assessment of the Kidney Donor Profile Index in a European cohort. Nephrol Dialys Transplant. 2018 Aug;33(8):1465-72.. In our study we also observed a lower GFR in different KDPI groups, but we included in the analysis only functioning grafts. The GFR decrease at 5 years was not observed, probably due to the fact that the patients with worse graft function lost the graft before 5 years, therefore less patients had GFR measured at 5 years.
The other independent risk factors for graft loss, namely DSA and acute rejection episodes, were defined in other studies3333 Aubert O, Kamar N, Vernerey D, Viglietti D, Martinez F, Duong-Van-Huyen JP, et al. Long term outcomes of transplantation using kidneys from expanded criteria donors: prospective, population based cohort study. BMJ. 2015 Jul;351:h3557.
34 Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-50 mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol. 2013 Jun;28(4):148-53.
35 Gloor J, Winters J, Cornell L, Fix L, DeGoey S, Knauer R, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010 Mar;10(3):582-9.
36 Hoshino J, Everly MJ, Kaneku H, Ubara Y, Takaichi K, Terasaki PI. Impact of the presence and duration of donor-specific antibodies on renal function. Transplant Proc. 2014 Jan/Feb;46(1):75-80.-3737 Willicombe M, Brookes P, Santos-Nunez E, Galliford J, Ballow A, McLean A, et al. Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2011 Mar;11(3):470-7..
The mean cold ischemia time was around 24 h, similar to other studies in Brazil3838 Baptista APM, Silva Junior HT, Pestana JOM. Influência da manutenção hemodinâmica do doador falecido na função renal do receptor de transplante renal. J Bras Nefrol. 2013;35(4):289-98.,3939 Silva Junior HT, Offerni JCM, Carneiro VA, Paula MI, Neto ED, Lemos FBC, et al. Randomized trial of machine perfusion versus cold storage in recipients of deceased donor kidney transplants with high incidence of delayed graft function. Transplant Direct. 2017 Apr;3(5):e155., but superior to the US (14.2 to 17.9 h)4040 Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016 Jun;16(6):1834-47. and Europe (18 h)4141 Balaz P, Rokosny S, Wohlfahrtova M, Wohlfahrt P, Bartonova A, Pokorna E, et al. Identification of expanded-criteria donor kidney grafts at lower risk of delayed graft function. Transplantation. 2013 Oct;96(7):633-8.,4242 Pretagostini R, Lai Q, Poli L, Sandri GL, Travaglia D, Rossi M, et al. Predictive characteristics of delayed graft function after expanded and standard criteria donor kidney transplantations. Transplant Proc. 2009 May;41(4):1149-51. reports. In our sample, 21.3% of transplants had CIT superior to 24 h, which may contribute to our high rate of DGF (63.5%). This DGF rate is similar to the ones obtained in the other Brazilian studies, ranging from 54.2% to 70.8%1111 Helfer MS, Vicari AR, Spuldaro F, Gonçalves LF, Manfro RC. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation in a Brazilian center. Transplant Proc. 2014 Jul/Aug;46(6):1727-9.
12 Sandes-Freitas TV, Felipe CR, Aguiar WF, Cristelli MP, Tedesco-Silva H, Medina-Pestana JO. Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals. PLoS One. 2015 Dec;10(12):e0144188.-1313 Mota LS, Oliveira CMS, Pinheiro Junior FML, Santos LCO, Nóbrega DG, et al. Comparative study between kidney transplantation with expanded criteria deceased donor and standard criteria donor in a single center in Brazil. J Bras Nefrol. 2016;38:348-57.,4343 Oliveira CMC, Gomes DM, Reuber C, Santos DCO, Brito MLM, Oliveira PCBF, et al. Terapia de indução com timoglobulina versus anticorpo monoclonal anti-IL2R - uma análise pareada em transplante renal com doador falecido. J Bras Transplantes. 2011;14(2):1518-22., but much higher than presented in international studies3333 Aubert O, Kamar N, Vernerey D, Viglietti D, Martinez F, Duong-Van-Huyen JP, et al. Long term outcomes of transplantation using kidneys from expanded criteria donors: prospective, population based cohort study. BMJ. 2015 Jul;351:h3557.,4040 Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016 Jun;16(6):1834-47.,4444 Moers C, Smits JM, Maathuis MH, Treckmann J, Van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009 Jan;360(1):7-19.,4545 Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, et al. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014 Feb;14(2):404-15.. The CIT and DGF were not independent risk factors for graft loss but they could have contributed for the very high rate of acute rejection in our sample.
The strength of our study was to include a large number of kidney recipients while a retrospective single-center report was its limitation.
The predictive power of KDPI in our sample was moderated with an AUC-ROC of 0.577 (0.514-0.641; p = 0.027), which is similar to the results in the literature (C Statistic = 0.60 in the US). Thus, it is not precise enough to estimate with high confidence the quality of donors with close KDPI scores. Furthermore, the KDPI does not include other donor risk factors related to outcomes as pre-implant biopsies and kidney anatomical alterations (damage, atherosclerotic lesions, and cysts). The KDPI also does not include the probability of cancer and infection transmissions, except hepatitis C. In our sample there was no donor with cardiac death because in our country it is not allowed.
Conclusion
In our study, 36.6% of kidney donors were classified as ECDs and 28.8% had KDPI ≥ 85%. There was an overlap of ECD/SCD with KDPI scores between 60 and 95%. All KDPI scores above 95% were considered as ECDs.
The KDPI score showed a moderate accuracy to predict graft survival at 5 years. For each KDPI increment point there was an increased graft loss risk of 1%. Recipient immunological variables were more accurate to predict graft survival.
The GFR was significantly lower for higher KDPI scores at one and five years after transplantation. The KDPI calculator is available for free online and does not cause any delay in kidney allocation. It seems to be a useful tool to help in organ allocation, even though it was developed based only on the North American population.
Ideally, there should be an equation based on Brazilian donors to apply in our population.
References
-
1McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991-2001. Nephrol Dial Transplant. 2002 Dec;17(12):2212-9.
-
2Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001 Mar;12(3):589-97.
-
3Pérez-Sáez M, Arcos E, Comas J, Crespo M, Lloveras J, Pascual J, et al. Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: a time-dependent analysis. Am J Transplant. 2016;16(9):2724-33.
-
4Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009 Jul;88(2):231-6.
-
5Organ Procurement and Transplantation Network (OPTN). US Department of Health and Human Services. Data Reports - Regional Data [Internet]. USA: HHS; 2014; [cited 2016 May 4]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/
» https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/ -
6Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002 Nov;74(9):1281-6.
-
7Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150(9):604-12.
-
8International Business Machines (IBM). IBM SPSS statistics for Windows. Version 21. Armonk, NY: IBM Corp.; 2013.
-
9Rege A, Irish B, Castleberry A, Vikraman D, Sanoff S, Ravindra K, et al. Trends in usage and outcomes for expanded criteria donor kidney transplantation in the United States characterized by kidney donor profile index. Cureus. 2016 Nov;8(11):e887.
-
10Arias-Cabrales C, Pérez-Sáez MJ, Redondo-Pachón D, Buxeda A, Burballa C, Bermejo S, et al. Usefulness of the KDPI in Spain: a comparison with donor age and definition of standard/expanded criteria donor. Nefrologia. 2018 Sep/Oct;38(5):503-13.
-
11Helfer MS, Vicari AR, Spuldaro F, Gonçalves LF, Manfro RC. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation in a Brazilian center. Transplant Proc. 2014 Jul/Aug;46(6):1727-9.
-
12Sandes-Freitas TV, Felipe CR, Aguiar WF, Cristelli MP, Tedesco-Silva H, Medina-Pestana JO. Prolonged delayed graft function is associated with inferior patient and kidney allograft survivals. PLoS One. 2015 Dec;10(12):e0144188.
-
13Mota LS, Oliveira CMS, Pinheiro Junior FML, Santos LCO, Nóbrega DG, et al. Comparative study between kidney transplantation with expanded criteria deceased donor and standard criteria donor in a single center in Brazil. J Bras Nefrol. 2016;38:348-57.
-
14Woodside KJ, Merion RM, Leichtman AB, Santos R, Arrington CJ, Rao PS, et al. Utilization of kidneys with similar kidney donor risk index values from standard versus expanded criteria donors. Am J Transplant. 2012 Jun;12(8):2106-14.
-
15Molnar MZ, Streja E, Kovesdy CP, Shah A, Huang E, Bunnapradist S, et al. Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes. Am J Kidney Dis. 2012 Jun;59(6):841-8.
-
16Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011 May;11(5):1025-30.
-
17Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;(3 Suppl 4):114-25.
-
18Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2(8):701-11.
-
19Sung RS, Guidinger MK, Leichtman AB, Lake C, Metzger RA, Port FK, et al. Impact of the expanded criteria donor allocation system on candidates for and recipients of expanded criteria donor kidneys. Transplantation. 2007 Nov;84(9):1138-44.
-
20Mezrich JD, Pirsch JD, Fernandez LA, Foley DP, Bellingham JM, Odorico JS, et al. Differential outcomes of expanded-criteria donor renal allografts according to recipient age. Clin J Am Soc Nephrol. 2012 Jul;7(7):1163-71.
-
21Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 2008 Sep;52(3):553-86.
-
22Port FK, Dykstra DM, Merion RM, Wolfe RA. Trends and results for organ donation and transplantation in the United States, 2004. Am J Transplant. 2005 Apr;5(4 Pt 2):843-9.
-
23Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093-109.
-
24Gandolfini I, Buzio C, Zanelli P, Palmisano A, Cremaschi E, Vaglio A, et al. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014 Nov;14(11):2515-25.
-
25Han M, Jeong JC, Koo TY, Jeon HJ, Kwon HY, Kim YJ, et al. Kidney donor risk index is a good prognostic tool for graft outcomes in deceased donor kidney transplantation with short, cold ischemic time. Clin Transplant. 2014;28(3):337-44.
-
26Lehner LJ, Kleinsteuber A, Halleck F, Khadzhynov D, Schrezenmeier E, Duerr M, et al. Assessment of the Kidney Donor Profile Index in a European cohort. Nephrol Dialys Transplant. 2018 Aug;33(8):1465-72.
-
27Snyder J, Salkowski N, Wey A, Israni AK, Schold JD, Segev DL, et al. Effects of high-risk kidneys on scientific registry of transplant recipients program quality reports. Am J Transplant. 2016 Sep;16(9):2646-53.
-
28Peters-Sengers H, Heemskerk MBA, Geskus RB, Kers J, Van Der Heide JJH, Berger SP, et al. Validation of the prognostic Kidney Donor Risk Index scoring system of deceased donors for renal transplantation in the Netherlands. Transplantation. 2018 Jan;102(1):162-70.
-
29Martín RMGM, Díaz JAR, Molina MC, Tornel BMC, Soto JB, Ortega AO, et al. Validation of KDRI/KDPI for the selection of expanded criteria kidney donors. Nefrologia. 2018 May/Jun;38(3):297-303.
-
30Bae S, Massie AB, Luo X, Anjum S, Desai NM, Segev DL. Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI). Am J Transplant. 2016 Jul;16(7):2202-7.
-
31Eurotransplant International Foundation (EIF). Eurotransplant Annual Report 2014 [Internet]. Netherlands: EIF; 2014. Disponível em: https://www.eurotransplant.org/cms/mediaobject.php?file=ar_2014.pdf
» https://www.eurotransplant.org/cms/mediaobject.php?file=ar_2014.pdf -
32Registro Brasileiro de Transplantes (RBT). 2017 - Dimensionamento dos Transplantes no Brasil e em cada estado. São Paulo: ABTO; 2018; XXIII(4):1-104. Disponível em: http://www.abto.org.br/abtov03/Upload/file/RBT/2017/rbt-imprensa-leitura-compressed.pdf
» http://www.abto.org.br/abtov03/Upload/file/RBT/2017/rbt-imprensa-leitura-compressed.pdf -
33Aubert O, Kamar N, Vernerey D, Viglietti D, Martinez F, Duong-Van-Huyen JP, et al. Long term outcomes of transplantation using kidneys from expanded criteria donors: prospective, population based cohort study. BMJ. 2015 Jul;351:h3557.
-
34Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-50 mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol. 2013 Jun;28(4):148-53.
-
35Gloor J, Winters J, Cornell L, Fix L, DeGoey S, Knauer R, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010 Mar;10(3):582-9.
-
36Hoshino J, Everly MJ, Kaneku H, Ubara Y, Takaichi K, Terasaki PI. Impact of the presence and duration of donor-specific antibodies on renal function. Transplant Proc. 2014 Jan/Feb;46(1):75-80.
-
37Willicombe M, Brookes P, Santos-Nunez E, Galliford J, Ballow A, McLean A, et al. Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2011 Mar;11(3):470-7.
-
38Baptista APM, Silva Junior HT, Pestana JOM. Influência da manutenção hemodinâmica do doador falecido na função renal do receptor de transplante renal. J Bras Nefrol. 2013;35(4):289-98.
-
39Silva Junior HT, Offerni JCM, Carneiro VA, Paula MI, Neto ED, Lemos FBC, et al. Randomized trial of machine perfusion versus cold storage in recipients of deceased donor kidney transplants with high incidence of delayed graft function. Transplant Direct. 2017 Apr;3(5):e155.
-
40Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016 Jun;16(6):1834-47.
-
41Balaz P, Rokosny S, Wohlfahrtova M, Wohlfahrt P, Bartonova A, Pokorna E, et al. Identification of expanded-criteria donor kidney grafts at lower risk of delayed graft function. Transplantation. 2013 Oct;96(7):633-8.
-
42Pretagostini R, Lai Q, Poli L, Sandri GL, Travaglia D, Rossi M, et al. Predictive characteristics of delayed graft function after expanded and standard criteria donor kidney transplantations. Transplant Proc. 2009 May;41(4):1149-51.
-
43Oliveira CMC, Gomes DM, Reuber C, Santos DCO, Brito MLM, Oliveira PCBF, et al. Terapia de indução com timoglobulina versus anticorpo monoclonal anti-IL2R - uma análise pareada em transplante renal com doador falecido. J Bras Transplantes. 2011;14(2):1518-22.
-
44Moers C, Smits JM, Maathuis MH, Treckmann J, Van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009 Jan;360(1):7-19.
-
45Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, et al. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014 Feb;14(2):404-15.
Publication Dates
-
Publication in this collection
11 May 2020 -
Date of issue
Apr-Jun 2020
History
-
Received
21 Dec 2018 -
Accepted
03 Dec 2019