Abstract
Vascular calcification decreases compliance and increases morbidity. Mechanisms of this process are unclear. The role of oxidative stress and effects of antioxidants have been poorly explored. We investigated effects of the antioxidants lipoic acid (LA) and tempol in a model of atherosclerosis associated with elastocalcinosis. Male New Zealand white rabbits (2.5-3.0 kg) were fed regular chow (controls) or a 0.5% cholesterol (chol) diet+104 IU/day vitamin D2 (vitD) for 12 weeks, and assigned to treatment with water (vehicle, n=20), 0.12 mmol·kg-1·day-1 LA (n=11) or 0.1 mmol·kg-1·day-1 tempol (n=15). Chol+vitD-fed rabbits developed atherosclerotic plaques associated with expansive remodeling, elastic fiber disruption, medial calcification, and increased aortic stiffness. Histologically, LA prevented medial calcification by ∼60% and aortic stiffening by ∼60%. LA also preserved responsiveness to constrictor agents, while intima-media thickening was increased. In contrast to LA, tempol was associated with increased plaque collagen content, medial calcification and aortic stiffness, and produced differential changes in vasoactive responses in the chol+vitD group. Both LA and tempol prevented superoxide signals with chol+vitD. However, only LA prevented hydrogen peroxide-related signals with chol+vitD, while tempol enhanced them. These data suggest that LA, opposite to tempol, can minimize calcification and compliance loss in elastocalcionosis by inhibition of hydrogen peroxide generation.
Antioxidants; Vascular calcification; Atherosclerosis; Oxidative stress; Lipoic acid
Introduction
Medial layer vascular calcification and elastocalcinosis decrease arterial compliance and increase morbidity in patients with chronic renal disease, diabetes and other conditions, including aging (11. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 2009; 5: 185-197, doi: 10.2147/VHRM.S4822.
https://doi.org/10.2147/VHRM.S4822...
). The mechanisms involved in the pathogenesis of these processes are incompletely understood, but recent research on vascular inflammation has shown that calcification is not simply a passive process, but an active, immune-mediated process (22. Stinghen AE, Bucharles S, Riella MC, Pecoits-Filho R. Immune mechanisms involved in cardiovascular complications of chronic kidney disease. Blood Purif 2010; 29: 114-120, doi: 10.1159/000245636.
https://doi.org/10.1159/000245636...
). Accordingly, another possible factor involved in vascular calcification is oxidative stress. Oxidative stress might result not only from increased inflammation, but also from other factors, including abnormal signaling of calcifying vascular cells (33. Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, et al. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci 2007; 1117: 40-50, doi: 10.1196/annals.1402.075.
https://doi.org/10.1196/annals.1402.075...
) and endoplasmic reticulum stress (44. Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 2009; 11: 2409-2427, doi: 10.1089/ars.2009.2625.
https://doi.org/10.1089/ars.2009.2625...
). These characteristics highlight the usual background of atherosclerotic changes in which calcification develops. We recently characterized the role of oxidative stress in the progression of aortic valve calcification (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
), while investigating the effects of tempol and lipoic acid (LA). These agents have distinct mechanisms of redox modulation. The former has been described as a superoxide dismutase mimetic that also diverts protein nitration to nitrosation (66. Fernandes DC, Medinas DB, Alves MJ, Augusto O. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic Biol Med 2005; 38: 189-200, doi: 10.1016/j.freeradbiomed.2004.09.027.
https://doi.org/10.1016/j.freeradbiomed....
). The latter has complex effects that include induction of antioxidant defense enzymes via nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (77. Ogborne RM, Rushworth SA, O'Connell MA. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler Thromb Vasc Biol 2005; 25: 2100-2105, doi: 10.1161/01.ATV.0000183745.37161.6e.
https://doi.org/10.1161/01.ATV.000018374...
). Thus, while tempol appears to directly interact with free radicals, LA appears mainly to interfere with redox signaling pathways. Experimentally reported effects of tempol include hydrogen peroxide-mediated vasorelaxation (88. Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 2007; 293: H2085-H2092, doi: 10.1152/ajpheart.00968.2006.
https://doi.org/10.1152/ajpheart.00968.2...
) and lowering of blood pressure (99. Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 2007; 293: H3246-H3253, doi: 10.1152/ajpheart.00957.2007.
https://doi.org/10.1152/ajpheart.00957.2...
). LA can prevent hypertension/hyperglycemia (1010. Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens 2003; 16: 173-179, doi: 10.1016/S0895-7061(02)03253-3.
https://doi.org/10.1016/S0895-7061(02)03...
) and atherosclerosis (1111. Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice. Circulation 2008; 117: 421-428, doi: 10.1161/CIRCULATIONAHA.107.725275.
https://doi.org/10.1161/CIRCULATIONAHA.1...
). Recent data indicate that LA was ineffective in prevention of warfarin-induced medial elastocalcinosis in rats (1212. Lalaoui MZ, El Midaoui A, de Champlain J, Moreau P. Is there a role for reactive oxygen species in arterial medial elastocalcinosis? Vascul Pharmacol 2007; 46: 201-206, doi: 10.1016/j.vph.2006.10.006.
https://doi.org/10.1016/j.vph.2006.10.00...
), but did prevent vitamin D3-induced calcification via preservation of mitochondrial function and restoration of the Gas6/Axl/Akt survival pathway (1313. Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR, et al. Alpha-lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med 2012; 16: 273-286, doi: 10.1111/j.1582-4934.2011.01294.x.
https://doi.org/10.1111/j.1582-4934.2011...
). Both tempol and LA prevent endothelial dysfunction in animal models of vascular disease (1414. Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res 2003; 37: 33-40, doi: 10.1080/1071576021000028442.
https://doi.org/10.1080/1071576021000028...
), and LA ameliorates endothelial dysfunction in humans with metabolic syndrome (1515. Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation 2005; 111: 343-348, doi: 10.1161/01.CIR.0000153272.48711.B9.
https://doi.org/10.1161/01.CIR.000015327...
). Here, we focused on the vascular effects of LA or tempol in a rabbit model of vascular/aortic valve calcification (1616. Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol 2003; 41: 1211-1217, doi: 10.1016/S0735-1097(03)00090-1.
https://doi.org/10.1016/S0735-1097(03)00...
) induced by vitamin D supplementation and a cholesterol-enriched diet. We evaluated the treatment effect on elastocalcinosis associated with atherosclerotic expansive remodeling.
Material and Methods
Reagents
All chemicals, including α-lipoic-acid, tempol, cholesterol, superoxide dismutase-polyethylene glycol (PEG-SOD) and PEG-catalase were from Sigma Chemical (USA); 2′,3′-dichlorofluorescein diacetate (DCF) and dihydroethidium (DHE) from Invitrogen (USA); OCT Tissue Tek embebbing compound from Fisher Scientific (USA). RAM11 antibody (dilution 1:200) was from Dako (Denmark). Immunohistochemical reactions were assayed using the Vectastain Elite ABC System (Vector, USA).
Rabbit model
This model was originally described by Drolet et al. (1616. Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol 2003; 41: 1211-1217, doi: 10.1016/S0735-1097(03)00090-1.
https://doi.org/10.1016/S0735-1097(03)00...
) as a model of aortic valve calcification. Male New Zealand white rabbits (2.5-3.0 kg) were fed either regular chow (controls, n=32) or 0.5% cholesterol+104 IU/day vitamin D2 (chol+vitD) (HCD, n=20). Additional chol+vitD-fed rabbits were given 0.12 mmol·kg-1·day-1 lipoic acid (n=11) or 0.1 mmol·kg-1·day-1 tempol (n=15) in drinking water. For comparison purposes, we also studied rabbits fed only a 0.5%-cholesterol diet (n=9). After 12 weeks, rabbits were anesthetized with 30 mg/kg ketamine and 3.5 mg/kg xylazine, im. Blood samples for total cholesterol, calcium, phosphorus, glucose, creatinine/urea were collected. Blood pressure was then measured via a catheter that was inserted through the carotid artery and was advanced until reaching the thoracic aorta. After euthanasia with a lethal dose of 100 mg/kg pentobarbital sodium, the descending thoraco-abdominal aorta was collected for further analysis. Most rabbits used in our study were also part of another parallel study focusing on aortic valve calcification (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
). This study was approved by an internal scientific institutional committee and by the Ethics Committee of Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (CAPPESQ #254/03), and complied with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, rev. 1996).
Vascular ultrasound and radiofrequency measurements
In vivo images of subdiaphragmatic aorta were obtained by a non-invasive high-definition ultrasonography “echotracking” device (Wall-Track System 2, Pie Medical, The Netherlands) as described elsewhere (1717. Bortolotto LA, Hanon O, Franconi G, Boutouyrie P, Legrain S, Girerd X. The aging process modifies the distensibility of elastic but not muscular arteries. Hypertension 1999; 34: 889-892, doi: 10.1161/01.HYP.34.4.889.
https://doi.org/10.1161/01.HYP.34.4.889...
). Briefly, two consecutive images of the descending aorta were stored and converted to a radiofrequency signal in a computer. Automatic measurements of internal diameter, wall thickness and beat-to-beat distension (percentage systolic-diastolic variation in internal diameter) were measured. The accuracy of the system was 30 µm for the diastolic diameter measurement and <1 µm for the pulsatile change in diameter. Total vessel diameter (mm) was calculated as the lumen radius+(2×posterior wall thickness).
Aortic stiffness
Segments of descending thoracic aorta (5 mm) were incubated with 10 µM sodium nitroprusside in 0.9% NaCl, to avoid vascular smooth muscle tone. Then, segments were mounted on an organ-chamber setup and subjected to cumulative distention at a rate of 0.5-mm increments every 30 s, until measurable tension was detected. At this point, the strain (length of distention in mm) was plotted against incremental developed tension (i.e., each 0.5-mm strain vs tension in g). The slope of this line was taken as a measure of aortic stiffness (modified from Ref. 1818. Kingwell BA, Arnold PJ, Jennings GL, Dart AM. Spontaneous running increases aortic compliance in Wistar-Kyoto rats. Cardiovasc Res 1997; 35: 132-137, doi: 10.1016/S0008-6363(97)00079-5.
https://doi.org/10.1016/S0008-6363(97)00...
).
Vessel histology and macrophage detection
Formaldehyde-fixed aortic segments were embedded in paraffin and processed for histology with Von Kossa, Masson's trichrome, hematoxylin and eosin, and Verhoeff-Van Gieson staining. Macrophages were identified by RAM-11 antibody immunostaining. Collagen deposition, macrophage infiltration and calcification area were calculated in segments of descending thoracic aorta in a blinded manner using the Quantimet analysis software (Leica, Germany).
Organ chamber vascular ring experiments
Vascular reactivity was assessed as described previously (1919. Laurindo FR, Pedro MA, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, et al. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 1994; 74: 700-709, doi: 10.1161/01.RES.74.4.700.
https://doi.org/10.1161/01.RES.74.4.700...
). Briefly, 5-mm aortic rings were mounted in an organ chamber with Krebs solution (103 mM NaCl, 25 mM NaHCO3, 11 mM C6H12O6, 4.7 mM KCl, 1.9 mM CaCl2, 1.2 mM MgSO4, 1.06 mM KH2PO4), pH 7.4, 37°C, aerated with 95% O2 and 5% CO2, and attached to a force-displacement transducer (Mp100, Biopac, USA). For isometric tension recording, resting tension was set at 3 g for an equilibration period of 60 min. Precontraction with 0.1 µM noradrenaline was followed by testing endothelium-dependent relaxation with acetylcholine (Ach). Contraction in response to 0.12 M KCl and the NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 0.1 mM) was measured to assess maximal vascular contraction and basal NO production, respectively. Endothelium-independent relaxation was assessed with sodium nitroprusside (SNP). The relaxation curves were compared to assess maximum effect and half maximal effective concentration (EC50) dosing.
In situ aortic ROS microfluorotopography
In situ aortic ROS microfluorotopography was performed with DHE for superoxide and DCF for hydrogen peroxide, as detailed previously (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
). Rabbit aorta segments were cryo-cut (30 µm thickness) and incubated with 3 µM DHE or 3 µM DCF; images were obtained with a Zeiss Axiovert 100M scanning confocal microscope and the Axiovision software (Carl Zeiss, Germany). Parallel reading of images was performed with identical laser acquisition settings. The influence of superoxide or hydrogen peroxide on fluorescent signals was assessed via parallel slice incubation with 500 U/mL PEG-SOD or 400 U/mL PEG-catalase.
Statistical analysis
Parametric distribution of all variables was assessed with the Shapiro-Wilk test (all samples, <50 per group). Data with a normal distribution are reported as means±SE and were compared with one-way ANOVA, followed by the Student-Newman-Keuls test. For non-parametric distributions of data, variables are reported as box plots with median±interquartile range and range. These data were analyzed with the Kruskal-Wallis test followed by the Dunn test. Significance was considered when P<0.05.
Results
Plasma cholesterol, creatinine and calcium-phosphorus product were increased in all chol+vitD-fed rabbits. There were no significant changes in rabbits given tempol or LA. All values were similar to those reported previously (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
).
Lipoic acid prevents increase in vascular stiffness
While HCD rabbits developed macroscopic enlargement of the arterial tree at several levels, ultrasound/radiofrequency measurements indicated borderline significance for in vivo expansive remodeling (Figure 1). Such remodeling was due to increased wall (intimal+medial) thickness with unchanged lumen diameter. There was little change in distension (data not shown). LA-treated rabbits displayed significant expansive vascular remodeling due primarily to vascular wall thickening (Figure 1), contrary to tempol-treated rabbits.
Effects of tempol or lipoic acid on vascular remodeling assessed in rabbits using in vivo radiofrequency-coupled ultrasound after 12 weeks of treatment. C: controls (n=6); HCD: 0.5% cholesterol+vitamin D feeding (n=8); HCDT: HCD+tempol (n=8); HCDLA: HCD+lipoic acid (n=8). See Material and Methods for details. Data are reported as boxplots representing median, interquartile ranges, and range. *P<0.05 vs C (Dunn test).
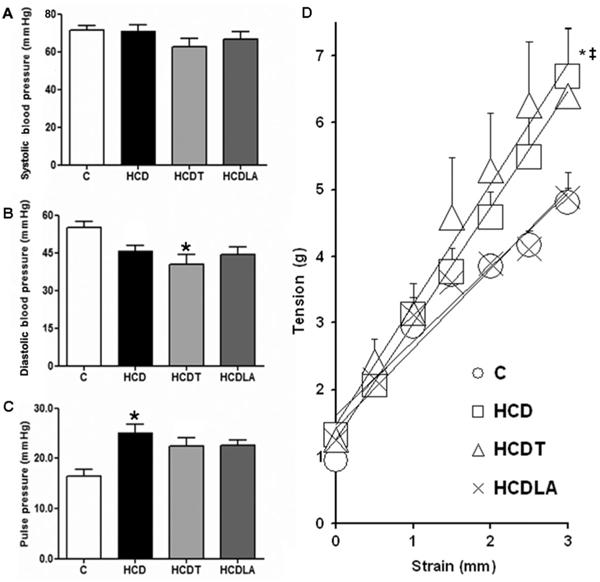
Pulse pressure, a surrogate marker of in vivo global arterial stiffness, was augmented in HCD rabbits and the observed increase was partially inhibited by LA or tempol treatment (Figure 2). However, experiments measuring in vitro aortic stiffness showed a significant increase in HCD rabbit aortas, which was unaltered by tempol treatment. In contrast, HCD lipoic acid rabbit aortas had stiffness levels similar to those from intact control rabbits.
A, B and C, Systolic blood pressure, diastolic blood pressure and pulse pressure measured with direct catheterization in anesthetized rabbits immediately before euthanasia. Heart rate was not different among groups. C: controls (n=28); HCD: 0.5% cholesterol+vitamin D feeding (n=16); HCDT: HCD+tempol (n=10); HCDLA: HCD+lipoic acid (n=7). D, In vitro arterial stiffness assessed as the slope of the strain-tension relationship in rabbit aortic segments. C (n=20); HCD (n=13); HCDT (n=13); HCDLA (n=10). See Material and Methods for details. Data are reported as means±SE. *P<0.05 vs C; ‡P<0.05 vs HCDLA (Student-Newman-Keuls test).
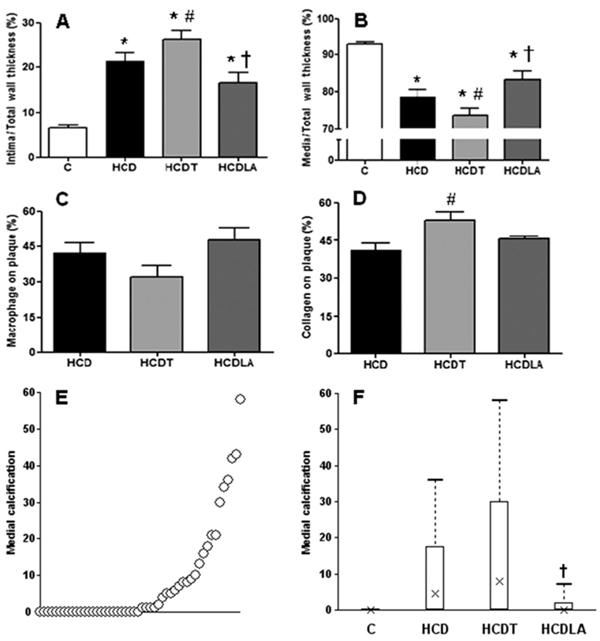
Lipoic acid, but not tempol, prevented vascular calcification
Histology confirmed the presence of atherosclerotic plaques in all rabbits given chol+vitD. The plaques were associated with massive calcium deposits and elastic fiber fragmentation/disorganization reminiscent of that observed in human elastocalcinosis in aging, diabetes or renal disease. Effects of tempol or LA in intima and media structures were quite diverse. Treatment with LA did not have any effect on thickness or collagen accumulation in the intima vs HCD rabbits. However, media thickness was significantly increased, in addition to marked prevention of calcification (Figure 3). There was also a non-significant increase (P=0.10) in plaque macrophage infiltration. In contrast, tempol induced further intimal growth associated with greater collagen accumulation and further enhancement of calcified nuclei in the media, in line with its reported increase in aortic valve calcification (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
). Of note, only 27% of aortas from LA-treated rabbits exhibited any measurable (>1% area) medial calcification at histology (P=NS vs controls). In contrast, HCD rabbit aortas showed a 69% incidence of medial calcification vs 73% with tempol. All groups showed similar collagen content in the media. Interestingly, plotting all values of medial calcification in HCD rabbits yielded a sigmoidal distribution, reminiscent of the pattern seen in human coronary calcification (2020. Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004; 24: 1161-1170, doi: 10.1161/01.ATV.0000133194.94939.42.
https://doi.org/10.1161/01.ATV.000013319...
).
Histomorphometrical analysis of arterial specimens. A, Intima/wall thickness ratio; B, media/wall thickness ratio; C, macrophage infiltration (%intimal area), assessed with monoclonal anti-rabbit macrophage antibody (RAM-11; 1:200); D, collagen accumulation (%intimal area), assessed with Masson's trichrome; E, plot in ascending order of medial calcification, detected by histomorphometry, for all rabbits, irrespective of treatment; F, medial layer calcification (%medial area), assessed with H&E and Von Kossa staining. C: controls (n=6 for A, B and F); HCD: 0.5% cholesterol+vitamin D feeding (n=16 for A, B and F, n=11 in C, and n=7 in D); HCDT: HCD+tempol (n=15 for A, B, and F, n=12 in C, and n=11 in D); HCDLA: HCD+lipoic acid (n=11 for A, B, and F, n=5 in C and D). See Material and Methods for details. In Panels A to D, data are reported as means±SE and parametric tests one-way ANOVA followed by the Student-Newman-Keuls test were used. In F, data are reported as boxplots representing median, interquartile ranges and range, and non-parametric tests Kruskal-Wallis followed by the Dunn test were used. *P<0.05 vs C; #P<0.05 vs HCD; †P<0.05 vs HCDT.
For comparison, rabbits given only a cholesterol-enriched diet had increased cholesterol plasma levels and atherosclerotic lesions at histology, but negligible calcification or expansive remodeling, with only minor (7%) wall thickening and unaltered vascular stiffness (data not shown). These findings confirmed that the model described by Drolet et al. (1616. Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol 2003; 41: 1211-1217, doi: 10.1016/S0735-1097(03)00090-1.
https://doi.org/10.1016/S0735-1097(03)00...
), but not rabbits fed cholesterol alone, is adequate for vascular calcification and stiffening studies, the focus of the present study.
Differential effects of lipoic acid and tempol on vascular contractility
Chol+vitD-fed rabbits had significantly impaired vascular contraction in response to noradrenaline. Remarkably, LA-treated rabbits had preserved contractility, similar to controls. In contrast, tempol significantly decreased the contraction induced by a single concentration of KCl, together with an augmented constriction response to L-NAME, suggestive of excessive basal NO production. While SNP-mediated relaxation was normal in all groups, neither LA nor tempol prevented the impaired Ach-mediated maximal relaxation observed in HCD rabbits. Interestingly, LA-treated rabbits had a lower sensitivity to Ach-mediated relaxation than the other chol+vitD-fed rabbits, without or with tempol supplementation. This was evident from higher EC50 values (median EC50 -6.47 vs -7.28 or -7.24 M, respectively, P<0.05). This is consistent with preservation of contractile capacity in LA-treated rabbits, thus requiring increased Ach concentrations for similar degrees of relaxation (Figure 4).
Organ-bath vasoreactivity studies in rabbit aortic rings. Contractions to 0.1 mM noradrenaline (A), 0.12 M KCl (B) or 0.1 mM L-NAME (C). Relaxation to acetylcholine (Ach) (D) or sodium nitroprusside (NPS) (E) are reported as %maximal contraction to agonist agents. C: controls (n=22); HCD: 0.5% cholesterol+vitamin D feeding (n=13); HCDT: HCD+tempol (n=12); HCDLA: HCD+lipoic acid (n=10). See Material and Methods for details. In A and B, data are reported as means±SE and parametric tests one-way ANOVA followed by the Student-Newman-Keuls test were used. In C, D, and E, data are reported as median±interquartile ranges, with range also reported in C, and non-parametric tests Kruskal-Wallis followed by the Dunn test were used. *P<0.05 vs C; #P<0.05 vs HCD; †P<0.05 vs HCDT; ##P<0.05 for HCDLA vs HCD or HCDT for EC50 of Ach-induced relaxation.
Lipoic acid or tempol differentially modulated redox processes
In HCD rabbits, DHE signals exhibited a patchy aspect with strong concentration mainly around medial calcification nuclei. Tempol robustly reduced DHE fluorescence to levels below even controls, but significantly increased DCF-derived signals, indicating increased H2O2 production, a result in line with previous data (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
,88. Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 2007; 293: H2085-H2092, doi: 10.1152/ajpheart.00968.2006.
https://doi.org/10.1152/ajpheart.00968.2...
). LA also partially decreased DHE-derived signals but, contrary to tempol, promoted a consistent decrease in DCF fluorescence, which was similar to controls (Figure 5). Control experiments with PEG-SOD and PEG-catalase revealed robust decreases in fluorescence signals with DHE and DCF, respectively (data not shown).
Upper panels, DHE fluorescence (red) in control and HCD-fed rabbits in the absence or presence of tempol or lipoic acid (LA). Please note increased signals around calcification nuclei (asterisks). Lower panels, DCF fluorescence (green) in control and 0.5% cholesterol+vitamin-fed rabbits in the absence or presence of tempol or LA. Controls with polyethylene glycol (PEG)-superoxide dismutase or PEG-catalase showed respective decreases in DHE or DCF signals (data not shown). C: controls; HCD: 0.5% cholesterol+vitamin D feeding; HCDT: HCD feeding+tempol; HCDLA: HCD feeding+LA; DCF: 2′,3′-dichlorofluorescein diacetate; DHE: dihydroethidium. Data are from 3 or more rabbits for each group. Magnification bar = 50 μm.
Discussion
In the present study, we showed that LA protected against medial vascular calcification and prevented the associated loss of arterial compliance and contractility, despite increasing arterial wall thickness. This increased artery wall thickness observed by ultrasound in LA-treated rabbits was due mainly to increased medial, rather than intimal, thickness as was demonstrated by histology. In contrast, tempol did not have such a preventive effect, and actually potentiated the increase in vascular calcification.
The HCD rabbit model, in addition to displaying aortic valve calcification (1616. Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol 2003; 41: 1211-1217, doi: 10.1016/S0735-1097(03)00090-1.
https://doi.org/10.1016/S0735-1097(03)00...
), also develops complex vascular disease including atherosclerosis with lumen diameter preservation (expansive remodeling), medial calcification and arterial stiffening. The vascular hypocontratility seen in the model could be explained either by a direct vitD effect (2121. Kahonen M, Nappi S, Jolma P, Hutri-Kahonen N, Tolvanen JP, Saha H, et al. Vascular influences of calcium supplementation and vitamin D-induced hypercalcemia in NaCl-hypertensive rats. J Cardiovasc Pharmacol 2003; 42: 319-328, doi: 10.1097/00005344-200309000-00002.
https://doi.org/10.1097/00005344-2003090...
) or medial calcification, with structural derangement of the medial layer. Such alterations, at least in part analogous to those described in human elastocalcinosis, differ substantially from those found in hypercholesterolemic rabbits, which do not display extensive calcification of the medial layer or vascular remodeling. These structural changes can also explain the differences in contraction responses to NA or KCl in this model, and those reported in the literature on hypercholesterolemic rabbits.
Multiple, opposite effects of different antioxidants should primarily reflect their different mechanisms of action. Tempol is a nitroxide that targets ROS and reactive nitrogen species indirectly (66. Fernandes DC, Medinas DB, Alves MJ, Augusto O. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic Biol Med 2005; 38: 189-200, doi: 10.1016/j.freeradbiomed.2004.09.027.
https://doi.org/10.1016/j.freeradbiomed....
), although its mechanism of action is debatable. The usually described SOD-mimetic activity may not be its major mechanism of action, which instead may involve a rapid reaction of tempol with nitrogen dioxide or carbonate radical (2222. Augusto O, Trindade DF, Linares E, Vaz SM. Cyclic nitroxides inhibit the toxicity of nitric oxide-derived oxidants: mechanisms and implications. An Acad Bras Cienc 2008; 80: 179-189, doi: 10.1590/S0001-37652008000100013.
https://doi.org/10.1590/S0001-3765200800...
). We recently reported that aortic segments from HCD rabbits showed increased Nox4, but not Nox1 NADPH oxidase isoform mRNA expression, and that tempol, but not LA, led to an even more pronounced increase in the expression of Nox4 (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
), an isoform associated mainly with H2O2 production (2323. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 2007; 406: 105-114, doi: 10.1042/BJ20061903.
https://doi.org/10.1042/BJ20061903...
). In our model, the final effect was a clear decrease of dihydroethidium fluorescence signals consistent with decreased superoxide output and, paradoxically, an increase in DCF-detectable oxidant, consistent at least in part with hydrogen peroxide. Contrarily, the mechanisms underlying LA effects do not seem to involve direct oxidant scavenging, considering the low rate constants of their direct reactions with this cyclic disulfide or its reduction product dihydrolipoic acid (2424. Trujillo M, Folkes L, Bartesaghi S, Kalyanaraman B, Wardman P, Radi R. Peroxynitrite-derived carbonate and nitrogen dioxide radicals readily react with lipoic and dihydrolipoic acid. Free Radic Biol Med 2005; 39: 279-288, doi: 10.1016/j.freeradbiomed.2005.03.014.
https://doi.org/10.1016/j.freeradbiomed....
). Rather, LA may target redox signaling indirectly via Nrf-2-dependent (77. Ogborne RM, Rushworth SA, O'Connell MA. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler Thromb Vasc Biol 2005; 25: 2100-2105, doi: 10.1161/01.ATV.0000183745.37161.6e.
https://doi.org/10.1161/01.ATV.000018374...
) transcriptional activation of antioxidant genes. Another relevant mechanism underlying LA effects could be its interference with endoplasmic reticulum stress, which is known to be associated with atherosclerosis and inflammation (44. Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 2009; 11: 2409-2427, doi: 10.1089/ars.2009.2625.
https://doi.org/10.1089/ars.2009.2625...
). In fact, LA decreased, while tempol increased, the nuclear expression of the proapoptotic transcription factor CHOP/GADD153 in cultured vascular smooth muscle cells exposed to the endoplasmic reticulum stressor tunicamycin (unpublished data from our laboratory). Therefore, the effects of both antioxidants regarding oxidant production may involve both direct and indirect pathways.
In our model, the final effect of LA was to decrease superoxide, and particularly hydrogen peroxide, signals. This further highlights a role for hydrogen peroxide in vascular dysfunction and calcification, which could reflect some known opposite effects of either species on vascular smooth muscle cell (VSMC) signaling, with superoxide associated mainly with proliferation and hydrogen peroxide with apoptosis (2525. Li PF, Dietz R, von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation 1997; 96: 3602-3609, doi: 10.1161/01.CIR.96.10.3602.
https://doi.org/10.1161/01.CIR.96.10.360...
). Moreover, recent observations showed that hydrogen peroxide plays a key role in VSMC osteogenic differentiation, via Akt-mediated induction of Runx2 transcription factor (2626. Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 2008; 283: 15319-15327, doi: 10.1074/jbc.M800021200.
https://doi.org/10.1074/jbc.M800021200...
). Our previous results in this model showed that tempol, but not LA, increased Nox4 expression (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
). This NADPH oxidase isoform appears to preferentially generate hydrogen peroxide rather than superoxide (2323. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 2007; 406: 105-114, doi: 10.1042/BJ20061903.
https://doi.org/10.1042/BJ20061903...
) and is associated with decreased VSMC proliferation and differentiation (2727. Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 2007; 27: 42-48, doi: 10.1161/01.ATV.0000251500.94478.18.
https://doi.org/10.1161/01.ATV.000025150...
). A peculiar aspect of our DCF signals (Figure 5) was their close association with elastic fibers. Interestingly, oxidative stress promotes (2828. Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res 2005; 66: 307-317, doi: 10.1016/j.cardiores.2005.01.012.
https://doi.org/10.1016/j.cardiores.2005...
) elastic network disorganization and elastocalcinosis. A role for oxidative stress in the calcification model is further corroborated by the effects of hydrogen peroxide and catalase addition in the in vitro calcification model (55. Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
https://doi.org/10.1161/ATVBAHA.107.1567...
).
Our data are in agreement with and extend the report of Kim et al. (1313. Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR, et al. Alpha-lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med 2012; 16: 273-286, doi: 10.1111/j.1582-4934.2011.01294.x.
https://doi.org/10.1111/j.1582-4934.2011...
), who recently demonstrated that LA inhibited in vitro and vitamin D3-induced in vivo vascular calcification by recovering mitochondrial function and restoring the Gas6/Axl/Akt survival pathway. On the other hand, our results are at variance with findings by Lalaoui et al. (1212. Lalaoui MZ, El Midaoui A, de Champlain J, Moreau P. Is there a role for reactive oxygen species in arterial medial elastocalcinosis? Vascul Pharmacol 2007; 46: 201-206, doi: 10.1016/j.vph.2006.10.006.
https://doi.org/10.1016/j.vph.2006.10.00...
) and Yamada et al. (2929. Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res 2012; 27: 474-485, doi: 10.1002/jbmr.539.
https://doi.org/10.1002/jbmr.539...
). Lalaoui observed no effect of LA in a rat model of warfarin-induced medial elastocalcinosis, and Yamada et al. reported that tempol was able to reduce arterial calcification by ∼33% in uremic rats. Differences in species, doses and time of antioxidant administration are possible reasons for these contrasting data. Furthermore, specific aspects of each calcification model should be considered. Warfarin inhibits carboxylation-driven activation of the Matrix Gla protein, a natural inhibitor of vascular calcification, and thus exploits a specific pathway of the calcification process per se. In comparison, our vitD model explores several mechanisms present in complex human vascular disease that drive medial calcification, in particular, inflammation and oxidative stress. In the warfarin model used by Lalaoui, there was a small elevation of aortic superoxide, while hydrogen peroxide levels were not assessed. In the present study, there was a marked increase in oxidant generation, and while tempol inhibited superoxide generation, it could not prevent elastocalcinosis. These opposing effects of tempol suggest that distinct redox signaling pathways have divergent effects on vascular calcification. Comparable considerations can be made for the research by Yamada et al. (2929. Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res 2012; 27: 474-485, doi: 10.1002/jbmr.539.
https://doi.org/10.1002/jbmr.539...
). Overall, combining the production and elimination of diverse intermediates such as superoxide and hydrogen peroxide under the label of “ROS” or “oxidative stress” is actually not accurate. They have distinct physicochemical properties and reactivities, and can have opposite effects on basic cellular functions, e.g., apoptosis (2525. Li PF, Dietz R, von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation 1997; 96: 3602-3609, doi: 10.1161/01.CIR.96.10.3602.
https://doi.org/10.1161/01.CIR.96.10.360...
). Grouping compounds with distinct effects, such as tempol and LA, together under the label of “antioxidants” also seems an oversimplification. Specific effects of some compounds, such as the increase in hydrogen peroxide with tempol, may contribute in some cases to collateral signaling, which in our study translated into enhanced calcification.
Clinical studies with antioxidants have resulted in considerable controversy, with somewhat conflicting but largely negative results (3030. Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol 2008; 101: 14D-19D, doi: 10.1016/j.amjcard.2008.02.003.
https://doi.org/10.1016/j.amjcard.2008.0...
). In this context, our study has potential implications. First, the effect of LA indicates that redox processes have a mechanistic role in vascular calcification and that appropriate interventions to correct abnormal signaling can result in a novel perspective to control or limit medial calcinosis in a variety of clinical settings. A parallel implication is that, despite being grouped together as antioxidants, tempol and LA acted in distinct pathophysiological directions, and support an overall conclusion that the effect of a given “antioxidant” will be highly specific for each compound and situation, thus negating a putative “class effect” based solely on antioxidant properties.
Research supported by FAPESP (Projects #2004/13683-0 and #2009/54764-6), and CNPq-Instituto Milênio Redoxoma. E. Bassi, M.K. Martinatti and M. Liberman were supported by FAPESP (grants #2004/04791-3, #2005/57463-6 and #2003/08042-2, respectively).
References
-
1Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 2009; 5: 185-197, doi: 10.2147/VHRM.S4822.
» https://doi.org/10.2147/VHRM.S4822 -
2Stinghen AE, Bucharles S, Riella MC, Pecoits-Filho R. Immune mechanisms involved in cardiovascular complications of chronic kidney disease. Blood Purif 2010; 29: 114-120, doi: 10.1159/000245636.
» https://doi.org/10.1159/000245636 -
3Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, et al. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci 2007; 1117: 40-50, doi: 10.1196/annals.1402.075.
» https://doi.org/10.1196/annals.1402.075 -
4Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 2009; 11: 2409-2427, doi: 10.1089/ars.2009.2625.
» https://doi.org/10.1089/ars.2009.2625 -
5Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 2008; 28: 463-470, doi: 10.1161/ATVBAHA.107.156745.
» https://doi.org/10.1161/ATVBAHA.107.156745 -
6Fernandes DC, Medinas DB, Alves MJ, Augusto O. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic Biol Med 2005; 38: 189-200, doi: 10.1016/j.freeradbiomed.2004.09.027.
» https://doi.org/10.1016/j.freeradbiomed.2004.09.027 -
7Ogborne RM, Rushworth SA, O'Connell MA. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler Thromb Vasc Biol 2005; 25: 2100-2105, doi: 10.1161/01.ATV.0000183745.37161.6e.
» https://doi.org/10.1161/01.ATV.0000183745.37161.6e -
8Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol 2007; 293: H2085-H2092, doi: 10.1152/ajpheart.00968.2006.
» https://doi.org/10.1152/ajpheart.00968.2006 -
9Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 2007; 293: H3246-H3253, doi: 10.1152/ajpheart.00957.2007.
» https://doi.org/10.1152/ajpheart.00957.2007 -
10Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens 2003; 16: 173-179, doi: 10.1016/S0895-7061(02)03253-3.
» https://doi.org/10.1016/S0895-7061(02)03253-3 -
11Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice. Circulation 2008; 117: 421-428, doi: 10.1161/CIRCULATIONAHA.107.725275.
» https://doi.org/10.1161/CIRCULATIONAHA.107.725275 -
12Lalaoui MZ, El Midaoui A, de Champlain J, Moreau P. Is there a role for reactive oxygen species in arterial medial elastocalcinosis? Vascul Pharmacol 2007; 46: 201-206, doi: 10.1016/j.vph.2006.10.006.
» https://doi.org/10.1016/j.vph.2006.10.006 -
13Kim H, Kim HJ, Lee K, Kim JM, Kim HS, Kim JR, et al. Alpha-lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med 2012; 16: 273-286, doi: 10.1111/j.1582-4934.2011.01294.x.
» https://doi.org/10.1111/j.1582-4934.2011.01294.x -
14Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res 2003; 37: 33-40, doi: 10.1080/1071576021000028442.
» https://doi.org/10.1080/1071576021000028442 -
15Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation 2005; 111: 343-348, doi: 10.1161/01.CIR.0000153272.48711.B9.
» https://doi.org/10.1161/01.CIR.0000153272.48711.B9 -
16Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol 2003; 41: 1211-1217, doi: 10.1016/S0735-1097(03)00090-1.
» https://doi.org/10.1016/S0735-1097(03)00090-1 -
17Bortolotto LA, Hanon O, Franconi G, Boutouyrie P, Legrain S, Girerd X. The aging process modifies the distensibility of elastic but not muscular arteries. Hypertension 1999; 34: 889-892, doi: 10.1161/01.HYP.34.4.889.
» https://doi.org/10.1161/01.HYP.34.4.889 -
18Kingwell BA, Arnold PJ, Jennings GL, Dart AM. Spontaneous running increases aortic compliance in Wistar-Kyoto rats. Cardiovasc Res 1997; 35: 132-137, doi: 10.1016/S0008-6363(97)00079-5.
» https://doi.org/10.1016/S0008-6363(97)00079-5 -
19Laurindo FR, Pedro MA, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, et al. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 1994; 74: 700-709, doi: 10.1161/01.RES.74.4.700.
» https://doi.org/10.1161/01.RES.74.4.700 -
20Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004; 24: 1161-1170, doi: 10.1161/01.ATV.0000133194.94939.42.
» https://doi.org/10.1161/01.ATV.0000133194.94939.42 -
21Kahonen M, Nappi S, Jolma P, Hutri-Kahonen N, Tolvanen JP, Saha H, et al. Vascular influences of calcium supplementation and vitamin D-induced hypercalcemia in NaCl-hypertensive rats. J Cardiovasc Pharmacol 2003; 42: 319-328, doi: 10.1097/00005344-200309000-00002.
» https://doi.org/10.1097/00005344-200309000-00002 -
22Augusto O, Trindade DF, Linares E, Vaz SM. Cyclic nitroxides inhibit the toxicity of nitric oxide-derived oxidants: mechanisms and implications. An Acad Bras Cienc 2008; 80: 179-189, doi: 10.1590/S0001-37652008000100013.
» https://doi.org/10.1590/S0001-37652008000100013 -
23Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 2007; 406: 105-114, doi: 10.1042/BJ20061903.
» https://doi.org/10.1042/BJ20061903 -
24Trujillo M, Folkes L, Bartesaghi S, Kalyanaraman B, Wardman P, Radi R. Peroxynitrite-derived carbonate and nitrogen dioxide radicals readily react with lipoic and dihydrolipoic acid. Free Radic Biol Med 2005; 39: 279-288, doi: 10.1016/j.freeradbiomed.2005.03.014.
» https://doi.org/10.1016/j.freeradbiomed.2005.03.014 -
25Li PF, Dietz R, von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation 1997; 96: 3602-3609, doi: 10.1161/01.CIR.96.10.3602.
» https://doi.org/10.1161/01.CIR.96.10.3602 -
26Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 2008; 283: 15319-15327, doi: 10.1074/jbc.M800021200.
» https://doi.org/10.1074/jbc.M800021200 -
27Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 2007; 27: 42-48, doi: 10.1161/01.ATV.0000251500.94478.18.
» https://doi.org/10.1161/01.ATV.0000251500.94478.18 -
28Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res 2005; 66: 307-317, doi: 10.1016/j.cardiores.2005.01.012.
» https://doi.org/10.1016/j.cardiores.2005.01.012 -
29Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res 2012; 27: 474-485, doi: 10.1002/jbmr.539.
» https://doi.org/10.1002/jbmr.539 -
30Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol 2008; 101: 14D-19D, doi: 10.1016/j.amjcard.2008.02.003.
» https://doi.org/10.1016/j.amjcard.2008.02.003
-
First published online January 24, 2014.
Publication Dates
-
Publication in this collection
Feb 2014
History
-
Received
4 May 2013 -
Accepted
7 Oct 2013