Trehalose induced drought tolerance in plants: physiological and molecular responses
DOI:
https://doi.org/10.15835/nbha50112584Keywords:
drought, osmolytes accumulation, oxidative stress photosynthesis, stress proteins, trehaloseAbstract
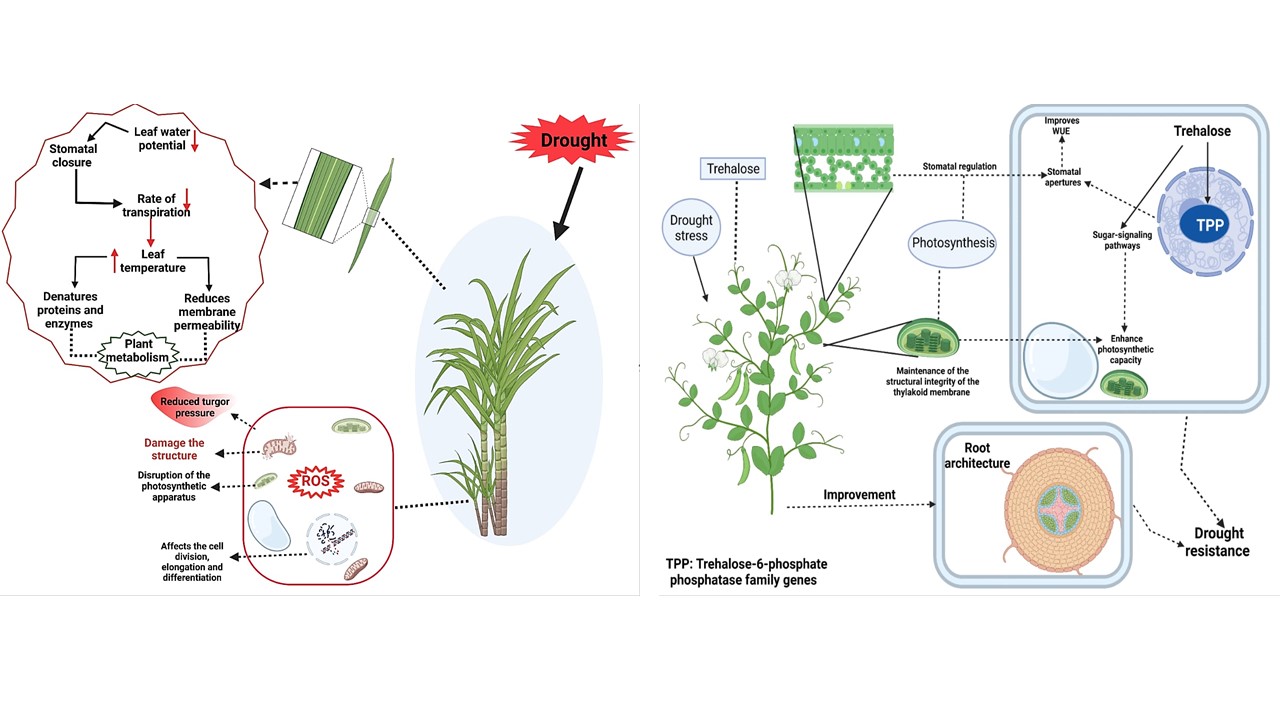
Drought stress is significant abiotic stress that limits crop growth and productivity across the globe. The intensity of drought stress continuously rises due to rapid climate change. Drought-induced alterations in physiological and bio-chemical processes by generating membrane dis-stability, oxidative stress, nutritional imbalance and leading to substantial reduction in growth and productivity. Plants accumulate various osmolytes that protect themselves from abiotic stresses' harmful effects. Trehalose (Tre) is a non-reducing sugar found in multiple microbes ranging from bacteria to yeast and in plants and it possesses an excellent ability to improve drought tolerance. Trehalose appreciably enhanced the plant growth, and counter the drought induced damages by maintaining cellular membranes, plant water relations, stomatal regulation, photosynthetic activities, nutrient uptake, osmolyte accumulation, activating stress proteins and detoxifying the reactive oxygen species (ROS) by strengthening the anti-oxidant system. Therefore, it is essential to understand the mechanism of exogenous and endogenous Tre in mitigating the drought-induced damages and to identify the potential research questions that must be answered in the future. Therefore, to better appraise the potential benefits of Tre in drought tolerance in this review, we discussed the diverse physiological and molecular mechanisms regulated by Tre under drought stress. We have a complete and updated picture on this topic to orientate future research directions on this topic.
References
Aamer M, Muhammad U, Li Z, Abid A, Su Q, Liu Y, … Huang G (2018). Foliar application of glycinebetaine (GB) alleviates the cadmium (Cd) toxicity in spinach through reducing Cd uptake and improving the activity of anti-oxidant system. Applied Ecology and Environmental Research 16:7575-7583. http://dx.doi.org/10.15666/aeer/1606_75757583
Abdallah MS, Abdelgawad Z, El-Bassiouny H (2016). Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. South African Journal of Botany 103:275-282. https://doi.org/10.1016/j.sajb.2015.09.019
Acosta-Pérez P, Camacho-Zamora BD, Espinoza-Sánchez EA, Gutiérrez-Soto G, Zavala-García F, Abraham-Juárez MJ, Sinagawa-García SR (2020). Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase genes and analysis of its differential expression in maize (Zea mays) seedlings under drought stress. Plants 9:315. https://doi.org/10.3390/plants9030315
Ahmad Z, Anjum S, Waraich EA, Ayub MA, Ahmad T, Tariq RMS, … Iqbal MA (2018). Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress–a review. Journal of Plant Nutrition 41:1734-1743. https://doi.org/10.1080/01904167.2018.1459688
Ahmed HE, Kord MA, Youssef H, Qaid EA (2016). Exogenous application of trehalose improves the physiological status of wheat cv. Giza 168 grown under stress. Egyptian Journal of Botany 56:627-646.
Ahmed HE, Youssef EA, Kord MA, Qaid EA (2013). Trehalose accumulation in wheat plant promotes sucrose and starch biosynthesis. Jordan Journal of Biological Sciences 6:143-149.
Akram N, Irfan I, Ashraf M (2016). Trehalose-induced modulation of antioxidative defence system in radish (Raphanus sativus L.) plants subjected to water-deficit conditions. Agrochimica 186-198.
Akram NA, Ashraf M (2011). Pattern of accumulation of inorganic elements in sunflower (Helianthus annuus L.) plants subjected to salt stress and exogenous application of 5-aminolevulinic acid. Pakistan Journal of Botany 43:521-530.
Akram NA, Ashraf M, Al-Qurainy F (2012). Aminolevulinic acid-induced changes in some key physiological attributes and activities of anti-oxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Scientia Horticulturae 142:143-148. https://doi.org/10.1016/j.scienta.2012.05.007
Akram NA, Noreen S, Noreen T, Ashraf M (2015). Exogenous application of trehalose alters growth, physiology and nutrient composition in radish (Raphanus sativus L.) plants under water-deficit conditions. Brazilian Journal of Botany 38:431-439. https://doi.org/10.1007/s40415-015-0149-7
Akram NA, Shafiq F, Ashraf M (2017). Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in Plant Science 8:613. https://doi.org/10.3389/fpls.2017.00613
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014). Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics 7:271-283. https://search.informit.org/doi/10.3316/informit.580352339413763
Aldesuquy H, Ghanem H (2015). Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by up-regulating membrane characteristics and anti-oxidant defense system. Journal of Horticulture. http://dx.doi.org/10.4172/2376-0354.1000139
Aldesuquy H, Ibraheem FL, Ghanem HE (2018). Assessment of salicylic acid and trehalose impact on root growth and water relations in relation to grain yield of drought wheat cultivars. Nutrition Food Science International 7:555701.
Ali Q, Ashraf M (2011). Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. Journal of Agronomy and Crop Science 197:258-271. https://doi.org/10.1016/j.foodchem.2005.08.016
Angioni A, Cabitza M, Russo MT, Caboni P (2006). Influence of olive cultivars and period of harvest on the contents of Cu, Cd, Pb, and Zn in virgin olive oils. Food Chemistry 99:525-529. https://doi.org/10.1111/j.1439-037X.2010.00463.x
Apel K, Hirt H (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55:373-399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Ashraf M (2009). Biotechnological approach of improving plant salt tolerance using anti-oxidants as markers. Biotechnology Advances 27:84-93. https://doi.org/10.1016/j.biotechadv.2008.09.003
Athari SN, Talebi R (2014). Effect of exogenous foliar salicylic acid application on sesame (Sesamum indicum L.) morphological characters, seed yield and oil content under different irrigation regimes. International Journal of Bioscienes 5:70-74. http://dx.doi.org/10.12692/ijb/5.9.70-8
Avonce N, Leyman B,Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G (2004). The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiology 136:3649-3659. https://doi.org/10.1104/pp.104.052084
Bae H, Herman E, Bailey B, Bae HJ, Sicher R (2005). Exogenous trehalose alters Arabidopsis transcripts involved in cell wall modification, abiotic stress, nitrogen metabolism, and plant defense. Physiologia Plantarum 125:114-126. https://doi.org/10.1111/j.1399-3054.2005.00537.x
Basu S, Ramegowda V, Kumar A, Pereira A (2016). Plant adaptation to drought stress. F1000 Research 5. https://dx.doi.org/10.12688%2Ff1000research.7678.1
Bijanzadeh E, Naderi R, Egan TP (2019). Exogenous application of humic acid and salicylic acid to alleviate seedling drought stress in two corn (Zea mays L.) hybrids. Journal of Plant Nutrition 42:1483-1495. https://doi.org/10.1080/01904167.2019.1617312
Campos CN, Ávila RG, de Souza KRD, Azevedo LM, Alves JD (2019). Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agricultural Water Management 211:37-47. https://doi.org/10.1016/j.agwat.2018.09.025
Cesaro A, De Giacomo O,Sussich F (2008). Water interplay in trehalose polymorphism. Food Chemistry 106:1318-1328. https://doi.org/10.1016/j.foodchem.2007.01.082
Chavoushi M, Najafi F, Salimi A, Angaji SA (2020). Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Scientia Horticulturae 259:108823. https://doi.org/10.1016/j.scienta.2019.108823
Chen TH, Murata N (2002). Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion in Plant Biology 5:250-257. https://doi.org/10.1016/S1369-5266(02)00255-8
Cherono S, Ntini C, Wassie M, Mollah MD, Belal MA, Ogutu C, Han Y (2021). Exogenous application of melatonin improves drought tolerance in coffee by regulating photosynthetic efficiency and oxidative damage. Journal of the American Society for Horticultural Science 146:24-32. https://doi.org/10.21273/JASHS04964-20
Crowe JH (2007). Trehalose as a “chemical chaperone”. Molecular aspects of the stress response: chaperones, membranes and networks 143-158. https://doi.org/10.1007/978-0-387-39975-1_13
Cruz RPD, Milach SCK (2004). Cold tolerance at the germination stage of rice: methods of evaluation and characterization of genotypes. Scientia Agricola 61:1-8. https://doi.org/10.1590/S0103-90162004000100001
Darvishan M, Tohidi-Moghadam HR, Zahedi H (2013). The effects of foliar application of ascorbic acid (vitamin C) on physiological and biochemical changes of corn (Zea mays L) under irrigation withholding in different growth stages. Maydica 58:195-200.
Dawood MG (2017). Improving drought tolerance of quinoa plant by foliar treatment of trehalose. Agricultural Engineering International: CIGR Journal 245-254.
Delorge I, Figueroa CM, Feil R, Lunn JE, Van Dijck P (2015). Trehalose-6-phosphate synthase 1 is not the only active TPS in Arabidopsis thaliana. Biochemical Journal 466:283-290. https://doi.org/10.1042/BJ20141322
Delorge I, Janiak M, Carpentier S, Van Dijck P (2014). Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Frontiers in Plant Science 5:147. https://doi.org/10.3389/fpls.2014.00147
Dias MC, Pinto DC, Figueiredo C, Santos C, Silva AM (2021). Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 185:112695. https://doi.org/10.1016/j.phytochem.2021.112695
Duman F, Aksoy A, Aydin Z, Temizgul R (2011). Effects of exogenous glycinebetaine and trehalose on cadmium accumulation and biological responses of an aquatic plant (Lemna gibba L.). Water, Air, & Soil Pollution 217:545-556. https://doi.org/10.1007/s11270-010-0608-5
Dykes L, Rooney L (2007). Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 52:105-111.
Eastmond PJ, Graham IA (2003). Trehalose metabolism: a regulatory role for trehalose-6-phosphate?. Current opinion in Plant Biology 6:231-235. https://doi.org/10.1016/S1369-5266(03)00037-2
Farooq M, Irfan M, Aziz T, Ahmad I, Cheema S (2013). Seed priming with ascorbic acid improves drought resistance of wheat. Journal of Agronomy and Crop Science 199:12-22. https://doi.org/10.1111/j.1439-037X.2012.00521.x
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009). Plant drought stress: effects, mechanisms and management. Sustainable Agriculture153-188. https://doi.org/10.1007/978-90-481-2666-8_12
Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C (2010). Trehalose and plant stress responses: friend or foe? Trends in Plant Science 15:409-417. https://doi.org/10.1146/annurev-arplant-050718-095929
Fichtner F, Lunn JE (2021). The role of trehalose 6-phosphate (Tre6P) in plant metabolism and development. Annual Review of Plant Biology 72. https://doi.org/10.1016/j.tplants.2010.04.004
Garg AK, Kim JK, Owens TG, Ranwala AP, Do Choi Y, Kochian LV, Wu RJ (2002). Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceedings of the National Academy of Sciences 99:15898-15903. https://doi.org/10.1073/pnas.252637799
Garg N, Manchanda G (2009). ROS generation in plants: boon or bane? Plant Biosystems 143:81-96. https://doi.org/10.1080/11263500802633626
Gill SS, Tuteja N (2010). Reactive oxygen species and anti-oxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48:909-930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA (2010). AtTPS1‐mediated trehalose 6‐phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. The Plant Journal 64:1-13. https://doi.org/10.1111/j.1365-313X.2010.04312.x
González JA, Gallardo M, Hilal MB, Rosa MD, Prado FE (2009). Physiological responses of quinoa (Chenopodium quinoa) to drought and waterlogging stresses: dry matter partitioning. Botantical Studies 50:35-42.
Guttikonda SK, Valliyodan B, Neelakandan AK, Tran LSP, Kumar R, Quach TN, … Pallardy SG (2014). Over-expression of AtDREB1D transcription factor improves drought tolerance in soybean. Molecular Biology Reports 41:7995-8008. Https://doi.org/10.1007/s11033-014-3695-3
Hafez E, Seleiman M (2017). Response of barley quality traits, yield and anti-oxidant enzymes to water-stress and chemical inducers. International Journal of Plant Production 11:477-490. https://doi.org/10.22069ijpp.2017.3712
Haghighi Z, Modarresi M, Mollayi S (2012). Enhancement of compatible solute and secondary metabolites production in Plantago ovata Forsk. by salinity stress. Journal of Medicinal Plants Research 6:3495-3500. https://doi.org/10.5897/JMPR12.159
Hammad SA, Ali OA (2014). Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Annals of Agricultural Sciences 59:133-145. https://doi.org/10.1016/j.aoas.2014.06.018
Harborne JB, Williams CA (2000). Advances in flavonoid research since 1992. Phytochemistry 55:481-504. https://doi.org/10.1016/S0031-9422(00)00235-1
Hasanuzzaman M, Bhuyan M, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019). Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384. https://doi.org/10.3390/antiox8090384
Hasanuzzaman M, Nahar K, Anee T, Khan M, Fujita M (2018). Silicon-mediated regulation of anti-oxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. South African Journal of Botany 115:50-57. https://doi.org/10.1016/j.sajb.2017.12.006
Hassan MU, Aamer M, Chattha MU, Ullah MA, Sulaman S, Nawaz M, … Guoqin H (2017). The role of potassium in plants under drought stress: Mini review. Journal of Basic and Applied Sciences 13:268-271.
Hassan MU, Aamer M, Chattha MU, Haiying T, Shahzad B, Barbanti L, … Guoqin H (2020). The critical role of zinc in plants facing the drought stress. Agriculture 10:396. https://doi.org/10.3390/agriculture10090.96
Hassan MU, Chattha MU, Khan I, Chattha MB, Aamer M, Nawaz M, … Khan TA (2019). Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environmental Science and Pollution Research 26:12673-12688. https://doi.org/10.1080/11263504.2020.1727987
Hassan MU, Chattha MU, Khan I, Chattha MB, Barbanti L, Aamer M, … Ali A (2021). Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies-A review. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology 155:211-234. https://doi.org/10.1080/11263504.2020.1727987
Horita M, Saruyama H (2006). Acceleration of germination of onion [Allium cepa] seeds by priming treatment with trehalose and raffinose. Horticultural Research 1:75-78. https://agris.fao.org/agris-search/search.do?recordID=JP2006006563
Hu Y, Schmidhalter U (2005). Drought and salinity: a comparison of their effects on mineral nutrition of plants. Journal of Plant Nutrition and Soil Science 168:541-549. https://doi.org/10.1002/jpln.200420516
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018). Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Frontiers in Plant Science 9:393. https://doi.org/10.3389/fpls.2018.00393
Ibrahim HA, Abdellatif YM (2016). Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Annals of Agricultural Sciences 61:267-274. https://doi.org/10.1016/j.aoas.2016.05.002
Jain NK, Roy I (2009). Effect of trehalose on protein structure. Protein Science 18:24-36. https://doi.org/10.1002/pro.3
Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R (2009). Drought stress in plants: a review on morphological characteristics and pigments composition. International Journal of Agriculture and Biology 11:100-105.
Jiang D, Chen W, Gao J, Yang F, Zhuang C (2019). Overexpression of the trehalose-6-phosphate phosphatase OsTPP3 increases drought tolerance in rice. Plant Biotechnology Reports 13:285-292. https://doi.org/10.1007/s11816-019-00541-4
Joo J, Choi HJ, Lee YH, Lee S, Lee CH, Kim CH, … Song SI (2014). Over-expression of BvMTSH, a fusion gene for maltooligosyltrehalose synthase and maltooligosyltrehalose trehalohydrolase, enhances drought tolerance in transgenic rice. BMB Reports 47:27. https://dx.doi.org/10.5483%2FBMBRep.2014.47.1
Joshi R, Sahoo KK, Singh AK, Anwar K, Pundir P, Gautam RK, … Singla-Pareek SL (2020). Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. Journal of Experimental Botany 71:653-668. https://doi.org/10.1093/jxb/erz462
Karim S, Aronsson H, Ericson H, Pirhonen M, Leyman B, Welin B, … Holmström K-O (2007). Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Molecular Biology 64:371-386. https://doi.org/10.1007/s11103-007-9159-6
Kaya C, Tuna AL, Ashraf M, Altunlu H (2007). Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environmental and Experimental Botany 60:397-403. https://doi.org/10.1016/j.envexpbot.2006.12.008
Khan I, Raza MA, Awan SA, Shah GA, Rizwan M, Ali B, … Brestic M (2020). Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, anti-oxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiology and Biochemistry 156:221-232. https://doi.org/10.1016/j.plaphy.2020.09.018
Khan SH, Ahmad N, Ahmad F, Kumar R (2010). Naturally occurring organic osmolytes: from cell physiology to disease prevention. IUBMB Life 62:891-895. https://doi.org/10.1002/iub.406
Khater M, Dawood MG, Sadak MS, Shalaby MA, El-Awadi M, El-Din KG (2018). Enhancement the performance of cowpea plants grown under drought conditions via trehalose application. Middle East Journal 7:782-800.
Khan N, Zandi P, Ali S, Mehmood A, Adnan SM, Yang J (2018). Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Frontiers in Microbiology 9:2507. https://doi.org/10.3389/fmicb.2018.02507
Kilic H, Yağbasanlar T (2010). The effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum ssp. durum) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38:164-170. https://doi.org/10.15835/nbha3814274
Kosar F, Akram N, Ashraf M (2015). Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. South African Journal of Botany 96:71-77. https://doi.org/10.1016/j.sajb.2014.10.015
Kosar F, Akram NA, Ashraf M, Ahmad A, Alyemeni MN, Ahmad P (2021). Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiologia Plantarum 172:317-333. https://doi.org/10.1111/ppl.13155
Kosar F, Akram NA, Ashraf M, Sadiq M, Al-Qurainy F (2018). Trehalose-induced improvement in growth, photosynthetic characteristics and levels of some key osmoprotectants in sunflower (Helianthus annuus L.) under drought stress. Pakistan Journal of Botany 50:955-961.
Kumar V, Rani A, Dixit AK, Bhatnagar D, Chauhan G (2009). Relative changes in tocopherols, isoflavones, total phenolic content, and antioxidative activity in soybean seeds at different reproductive stages. Journal of Agricultural and Food Chemistry 57:2705-2710. https://doi.org/10.1021/jf803122a
Li HW, Zang BS, Deng XW, Wang XP (2011). Over-expression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234:1007-1018. https://doi.org/10.1007/s00425-011-1458-0
Lin Q, Wang S, Dao Y, Wang J, Wang K (2020). Arabidopsis thaliana trehalose-6-phosphate phosphatase gene TPPI enhances drought tolerance by regulating stomatal apertures. Journal of Experimental Botany 71:4285-4297. https://doi.org/10.1093/jxb/eraa173
Lin Q, Yang J, Wang Q, Zhu H, Chen Z, Dao Y, Wang K (2019). Over-expression of the trehalose-6-phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana. BMC Plant Biology 19:1-15. https://doi.org/10.1186/s12870-019-1986-5
Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M (2014). Trehalose metabolism in plants. The Plant Journal 79:544-567. https://doi.org/10.1111/tpj.12509
Ma C, Wang Z, Kong B, Lin T (2013). Exogenous trehalose differentially modulates anti-oxidant defense system in wheat callus during water deficit and subsequent recovery. Plant Growth Regulation 70:275-285. https://doi.org/10.1007/s10725-013-9799-2
Mafakheri A, Siosemardeh A, Bahramnejad B, Struik P, Sohrabi Y (2010). Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian journal of crop science 4:580-585. https://doi.org/10.3316/informit.857341254680658
Maksimović IV, Kastori RR, Petrović NM, Kovačev LM, Sklenar PS (2003). The effect of water potential on accumulation of some essential elements in sugarbeet leaves, Beta vulgaris ssp. vulgaris. Zbornik Matice Srpske Za Prirodne Nauke 39-50. https://doi.org/10.2298/ZMSPN0304039M
Mehmood M, Khan I, Chattha M, Hussain S, Ahmad N, Aslam M, … Nawaz M (2021). Thiourea application protects maize from drought stress by regulating growth and physiological traits. Pakistan Journal of Science 75: 355-363.
Meng JF, Xu TF, Wang ZZ, Fang YL, Xi ZM, Zhang ZW (2014). The ameliorative effects of exogenous melatonin on grape cuttings under water‐deficient stress: anti-oxidant metabolites, leaf anatomy, and chloroplast morphology. Journal of Pineal Research 57:200-212. https://doi.org/10.1111/jpi.12159
Miranda JA, Avonce N, Suárez R, Thevelein JM, Van Dijck P, Iturriaga G (2007). A bifunctional TPS–TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 226:1411-1421. https://doi.org/10.1007/s00425-007-0579-y
Mostofa MG, Hossain MA, Fujita M (2015). Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of anti-oxidant defense and glyoxalase systems. Protoplasma 252:461-475. https://doi.org/10.1007/s00709-014-0691-3
Neha P, Kumar M, Solankey SS (2021). Impact of drought and salinity on vegetable crops and mitigation strategies. In: Advances in Research on Vegetable Production Under a Changing Climate. Springer, pp 235-253. https://doi.org/10.1007/978-3-030-63497-1_13
Nounjan N, Nghia PT, Theerakulpisut P (2012). Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate anti-oxidant enzymes and expression of related genes. Journal of Plant Physiology 169:596-604. https://doi.org/10.1016/j.jplph.2012.01.004
Nuccio ML, Wu J, Mowers R, Zhou HP, Meghji M, Primavesi LF, … Haque E (2015). Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nature Biotechnology 33:862-869. https://doi.org/10.1038/nbt.3277
Osakabe Y, Osakabe K, Shinozaki K, Tran LSP (2014). Response of plants to water stress. Frontiers in Plant Science 5:86. https://doi.org/10.3389/fpls.2014.00086
Parida AK, Dagaonkar VS, Phalak MS, Aurangabadkar LP (2008). Differential responses of the enzymes involved in proline biosynthesis and degradation in drought tolerant and sensitive cotton genotypes during drought stress and recovery. Acta Physiologiae Plantarum 30:619-627. https://doi.org/10.1007/s11738-008-0157-3
Paul S, Paul S (2014). Trehalose induced modifications in the solvation pattern of N-methylacetamide. The Journal of Physical Chemistry B 118:1052-1063. https://doi.org/10.1021/jp407782x
Pellny TK, Ghannoum O, Conroy JP, Schluepmann H, Smeekens S, Andralojc J, … Paul MJ (2004). Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnology Journal 2:71-82. https://doi.org/10.1111/j.1467-7652.2004.00053.x
Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J (2017). Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Frontiers in Plant Science 8:490. https://doi.org/10.3389/fpls.2017.00490
Pilon-Smits EA, Terry N, Sears T, Kim H, Zayed A, Hwang S, … Krutwagen RW (1998). Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. Journal of Plant Physiology 152:525-532. https://doi.org/10.1016/S0176-1617(98)80273-3
Ramon M, Rolland F, Thevelein JM, Van Dijck P, Leyman B (2007). ABI4 mediates the effects of exogenous trehalose on Arabidopsis growth and starch breakdown. Plant Molecular Biology 63:195-206. https://doi.org/10.1007/s11103-006-9082-2
Rasheed A, Ahmed S, Wassan GM, Solangi AM, Aamer M, Khanzada H, … Israr A (2018). Estimation of hybrid vigor for yield and yield related traits in tomato (Solanum lycopersicon MIll). International Journal of Bioscience 12(1):160-167. http://dx.doi.org/10.12692/ijb/12.1.160-167
Rasheed A, Ilyas M, Khan TN, Nawab NN, Ahmed I, Hussain MM, … Intikhab A (2017). Genetic Association and path coefficient analysis among yield and yield related traits in tomato (Solanum lycopersicon MILL.). International Journal of Biosciences 11(5):21-26. http://dx.doi.org/10.12692/ijb/11.5.21-26
Rasheed A, Tahir MM, Ilyas M (2019). An investigation on genetic variability for different quantitative and qualitative traits of wheat (Triticum aestivum L) genotypes. Gomal University Journal of Research 35(1):67-74.
Rasheed A, Fahad S, Hassan MU, Tahir MM, Aamer M, Wu, Z (2020a). A review on aluminum toxicity and quantitative trait loci mapping in rice (Oryza sativa L). Applied Ecology and Environmental Research 18:3951-3961. http://dx.doi.org/10.15666/aeer/1803_39513964
Rasheed A, Fahad S, Aamer M, Hassan MU, Tahir MM, Wu Z (2020b). Role of genetic factors in regulating cadmium uptake, transport and accumulation mechanisms and quantitative trait loci mapping in rice. a review. Applied Ecology and Environmental Research 18:4005-4023. http://dx.doi.org/10.15666/aeer/1803_40054023
Rasheed A, Gill RA, Hassan MU, Mahmood A, Qari S, Zaman QU, … Wu Z (2021a). A critical review: recent advancements in the use of crispr/cas9 technology to enhance crops and alleviate global food crises. Current Issues in Molecular Biology 43:1950-1976. https://doi.org/10.3390/cimb43030135
Rasheed A, Hassan M, Aamer M, Bian J, Xu Z, He X, Wu Z (2020). Iron toxicity, tolerance and quantitative trait loci mapping in rice; a review. Applied Ecology and Environmental Research 18:7483-7498. http://dx.doi.org/10.15666/aeer/1806_74837498
Rasheed A, Hassan MU, Fahad S, Aamer M, Batool M, Ilyas M, … Li H (2021b). Heavy metals stress and plants defense responses. In: Sustainable Soil and Land Management and Climate Change. CRC Press, pp 57-82.
Rasheed A, Wassan GM, Khanzada H, Solangi AM, Han R, Li H, … Wu Z (2021c). Identification of genomic regions at seedling related traits in response to aluminium toxicity using a new high-density genetic map in rice (Oryza sativa L.). Genetic Resources and Crop Evolution 68:1889-1903. https://doi.org/10.1007/s10722-020-01103-2
Richards A, Krakowka S, Dexter L, Schmid H, Wolterbeek A, Waalkens-Berendsen D, … Kurimoto M (2002). Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food and Chemical Toxicology 40:871-898. https://doi.org/10.1016/S0278-6915(02)00011-X
Rollins J, Habte E, Templer S, Colby T, Schmidt J, Von Korff M (2013). Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). Journal of Experimental Botany 64:3201-3212. https://doi.org/10.1093/jxb/ert158
Ruehr NK, Grote R, Mayr S, Arneth A (2019). Beyond the extreme: recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiology 39:1285-1299. https://doi.org/10.1093/treephys/tpz032
Sadak MS (2016). Mitigation of drought stress on fenugreek plant by foliar application of trehalose. International Journal of Chemistry Technology Research 9:147-155.
Sadak MS, El-Bassiouny HMS, Dawood MG (2019). Role of trehalose on anti-oxidant defense system and some osmolytes of quinoa plants under water deficit. Bulletin of the National Research Centre 43:5. https://doi.org/10.1186/s42269-018-0039-9
Schwarz S, Van Dijck P (2017). Trehalose metabolism: a sweet spot for Burkholderia pseudomallei virulence. Virulence 8:5-7. https://doi.org/10.1080/21505594.2016.1216295
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, … Battaglia ML (2021a). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. https://doi.org/10.3390/plants10020259
Seleiman MF, Aslam MT, Alhammad BA, Hassan MU, Maqbool R, Chattha MU, … Roy R (2021b). Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton International Journal of Experimental Botany. http://dx.doi.org/10.32604/phyton.2022.017365
Selote DS, Khanna-Chopra R (2010). Anti-oxidant response of wheat roots to drought acclimation. Protoplasma 245:153-163. https://doi.org/10.1007/s00709-010-0169-x
Sewelam N, Kazan K, Schenk PM (2016). Global plant stress signaling: reactive oxygen species at the cross-road. Frontiers in Plant Science 7:187. https://doi.org/10.3389/fpls.2016.00187
Shafiq S, Akram NA, Ashraf M (2015). Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Scientia Horticulturae 185:68-75. https://doi.org/10.1016/j.scienta.2015.01.010
Shahbaz M, Ashraf M, Athar HUR (2008). Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regulation 55:51-64. https://doi.org/10.1007/s10725-008-9262-y
Sharma P, Dubey RS (2005). Drought induces oxidative stress and enhances the activities of anti-oxidant enzymes in growing rice seedlings. Plant Growth Regulation 46:209-221. https://doi.org/10.1007/s10725-005-0002-2
Shemi R., Wang R., Gheith ESM, Hussain HA, Hussain S, Irfan M, … Wang L (2021). Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Scientific Reports 11:1-14. https://doi.org/10.1038/s41598-021-82264-7
Shi Y, Sun H, Wang X, Jin W, Chen Q, Yuan Z, Yu H (2019). Physiological and transcriptomic analyses reveal the molecular networks of responses induced by exogenous trehalose in plant. Plos One 14:e0217204. https://doi.org/10.1371/journal.pone.0217204
Singh D, Laxmi A (2015). Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Frontiers in Plant Science 6:895. https://doi.org/10.3389/fpls.2015.00895
Talaat NB, Shawky BT, Ibrahim AS (2015). Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environmental and Experimental Botany 113:47-58. https://doi.org/10.1016/j.envexpbot.2015.01.006
Teramoto N, Sachinvala ND, Shibata M (2008). Trehalose and trehalose-based polymers for environmentally benign, biocompatible and bioactive materials. Molecules 13:1773-1816. https://doi.org/10.3390/molecules13081773
Theerakulpisut P, Phongngarm S (2013). Alleviation of adverse effects of salt stress on rice seedlings by exogenous trehalose. Asian Journal of Crop Science 5:405-415.
Tiwari YK, Yadav SK (2020). Effect of high-temperature stress on ascorbate–glutathione cycle in maize. Agricultural Research 9:179-187. https://doi.org/10.1007/s40003-019-00421-x
Toscano S, Ferrante A, Romano D (2019). Response of Mediterranean ornamental plants to drought stress. Horticulturae 5:6. https://doi.org/10.3390/horticulturae5010006
Van Dijck P, Mascorro-Gallardo JO, De Bus M, Royackers K, Iturriaga G, Thevelein JM (2002). Truncation of Arabidopsis thaliana and Selaginella lepidophylla trehalose-6-phosphate synthase unlocks high catalytic activity and supports high trehalose levels on expression in yeast. Biochemical Journal 366:63-71. https://doi.org/10.1042/bj20020517
Wahid A, Gelani S, Ashraf M, Foolad MR (2007). Heat tolerance in plants: an overview. Environmental and Experimental Botany 61:199-223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang K, Li F, Gao M, Huang Y, Song Z (2020a). Mechanisms of trehalose-mediated mitigation of Cd toxicity in rice seedlings. Journal of Cleaner Production 267:121982. https://doi.org/10.1016/j.jclepro.2020.121982
Wang W, Chen Q, Xu S,Liu WC, Zhu X, Song CP (2020b). Trehalose‐6‐phosphate phosphatase E modulates ABA‐controlled root growth and stomatal movement in Arabidopsis. Journal of Integrative Plant Biology 62:1518-1534. https://doi.org/10.1111/jipb.12925
Waszczak C, Carmody M, Kangasjärvi J (2018). Reactive oxygen species in plant signaling. Annual Review of Plant Biology 69:209-236. https://doi.org/10.1146/annurev-arplant-042817-040322
Wingler A, Fritzius T, Wiemken A, Boller T, Aeschbacher RA (2000). Trehalose induces the ADP-glucose pyrophosphorylase gene, ApL3, and starch synthesis in Arabidopsis. Plant Physiology 124:105-114. https://doi.org/10.1104/pp.124.1.105
Xiong Q, Cao C, Shen T, Zhong L, He H, Chen X (2019). Comprehensive metabolomic and proteomic analysis in biochemical metabolic pathways of rice spikes under drought and submergence stress. Biochimica Biophysica Acta Proteins and Proteomics 1867:237-247. https://doi.org/10.1016/j.bbapap.2019.01.001
Yang L, Zhao X, Zhu H, Paul M, Zu Y, Tang Z (2014). Exogenous trehalose largely alleviates ionic unbalance, ROS burst, and PCD occurrence induced by high salinity in Arabidopsis seedlings. Frontiers in Plant Science 5:570. https://doi.org/10.3389/fpls.2014.00570
Yang X, Wang B, Chen L, Li P, Cao C (2019). The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Scientific Reports 9:1-12. https://doi.org/10.1038/s41598-019-40161-0
Zargar SM, Gupta N, Nazir M, Mahajan R, Malik FA, Sofi NR, … Salgotra R (2017). Impact of drought on photosynthesis: Molecular perspective. Plant Gene 11:154-159. https://doi.org/10.1016/j.plgene.2017.04.003
Zeid I (2009). Trehalose as osmoprotectant for maize under salinity-induced stress. Research Journal of Agriculture and Biological Sciences 5:613622.
Zlatev Z, Lidon FC (2012). An overview on drought induced changes in plant growth, water relationsand photosynthesis. Emirates Journal of Food and Agriculture 57-72.
Zoghi Z, Hosseini SM, Kouchaksaraei MT, Kooch Y, Guidi L (2019). The effect of biochar amendment on the growth, morphology and physiology of Quercus castaneifolia seedlings under water-deficit stress. European Journal of Forest Research 138:967-979. https://doi.org/10.1007/s10342-019-01217-y
Zulfiqar F, Akram NA, Ashraf M (2020). Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 251:1-17. https://doi.org/10.1007/s00425-019-03293-1
Zulfiqar F, Chen J, Finnegan PM, Younis A, Nafees M, Zorrig W, Hamed KB (2021). Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 10:1078. https://doi.org/10.3390/plants10061078
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Jinhua SHAO, Weixiong WU, Fahd RASUL, Hassan MUNIR, Kai HUANG, Masood I. AWAN, Tasahil S. ALBISHI, Muhammad ARSHAD, Qiliang HU, Guoqin HUANG, Muhammad U. HASSAN, Muhammad AAMER, Sameer H. QARI

This work is licensed under a Creative Commons Attribution 4.0 International License.
License:

Open Access Journal:
The journal allows the author(s) to retain publishing rights without restriction. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.










.png)







