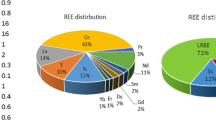
Abstract
Rare-earth element (REE) demand is expected to increase by a factor of up to 7 by 2040. Recycling avoids the significant hurdles associated with opening new mines, but collection and disassembly of REE-containing devices are barriers. Absolute and relative abundances of REEs and co-occurring constituents differ significantly in secondary compared to primary sources, presenting challenges and opportunities. REE concentrations are typically low, but manufactured devices include only the desired REE, avoiding the “REE balance problem” that besets natural ores. Fewer REEs need to be separated, as compared to separation of the entire lanthanide series. Co-recovery of precious (e.g., Au, Ag, Pt) or base metals (e.g., Cu, Sn, Zn) from e-wastes can offset recycling costs. Some examples of recently developed approaches for REE extraction and separation are presented here, with an emphasis on methods offering environmental benefits such as lower toxic chemical usage and reduced energy costs.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare-earth elements (REEs) are vital to the permanent magnets needed for electric vehicle (EV) motors and direct drive wind turbines and therefore their sustainable supply is necessary for the transition to a clean energy economy. Demand for REEs is expected to grow by 3 to 7 times between 2021 and 2040.1 The USGS estimated global production of REEs at 240,000 tonnes in 2020.2 Of that production, ~25% (Nd–Pr–Dy–Tb) of the mass is devoted to production of permanent magnets, which corresponds to nearly 80% of the economic value for REEs. Within the United States alone, the electric vehicle (EV) market is predicted to grow to 6.9 million vehicles by 2025 from 1.4 million in 2020.3 With a typical EV motor/generator requiring 2–5 kg of magnets, of which the REE can comprise about 1 kg, this could consume ~ 10% of the global supply of REEs used in magnet production. In the other major area of projected demand growth for permanent magnets, direct drive generators for wind energy, an average material intensity of 580.9 kg NdFeB per MW has been estimated.4 Direct drive generators are particularly desirable for offshore wind power because they require significantly less maintenance than gearbox turbine designs. Projected offshore wind power capacity in the United States is projected to grow to 86 GW by 2050,5 and this has been estimated to result in demand for >15 ktonnes of Nd for offshore US wind alone.4 The United States is not alone of course; for example, the European Union6 and Japan7 have acknowledged concerns about rising demand for magnet REEs as they pursue their own transitions to lower carbon energy systems.
Forecasts of steeply rising global demand for Nd and other REEs suggest that recycling will need to become a significant source of these critical metals, supplementing increased primary production.8 Currently, REE recycling from end-of-life (EOL) consumer products or industrial wastes is minimal, largely due to the difficult economics.9 Obstacles to recycling include inefficient (or nonexistent) collection, the high cost of dismantlement to retrieve the components containing the targeted elements, and lack of cost-effective methods for recovery from recycling feedstocks.10, 11 Other significant impediments are that many potential feedstocks have very low REE content to begin with, and available quantities of the feedstocks are not sufficient to warrant investment in recycling facilities.12 In some cases, the feedstocks’ EOL condition make it difficult to access the REE-containing components within them.
Nevertheless, because of the expected gap between supply and demand, the incentives for REE recycling are expected to increase, and considerable effort has been directed in recent years toward development of new approaches tailored for recovery from EOL products or secondary sources. The most promising feedstock targets are those that have the potential to provide significant quantities of critical rare earths, due to concentration and or quantity (ideally both), as well as cost-effective collection and pre-processing. Such feedstocks include permanent magnets from hard disk drives (HDDs), actuators such as low-profile speakers in TVs and mobile devices, and compact motor/generators, such as the traction motors of electric and hybrid vehicles. Industrial wastes, including swarfs (machining debris) and breakages from magnet manufacturing, or high volume spent catalysts, are also potential target sources. The most promising new approaches are specifically designed to take advantage of the special characteristics of recycling feedstock compositions, as compared with primary rare earth ores. One notable advantage is that recycling technologies avoid what is known as the REE “balance problem,” where the least valuable lanthanides, cerium and lanthanum, constitute the majority of the REEs in ores such as bastnäsite and monazite.13 This situation leads to high costs for recovery of the most valued REEs, because of the need to separate out and stockpile the less desired REEs.14 Manufactured products, by definition, contain only the desired elements, reducing the separation and excess production challenges. Another notable advantage is that they do not contain U and Th, radioactive and toxic elements that complicate the processing of many primary rare earth sources.15
Still, as noted by Gaustad et al., the absolute mass of targeted rare earths is small in most recycling feedstocks, compared with primary ores.12 If high REE content fractions can be separated out of electronic waste (e-waste) streams, the concentration of high-value REEs can be dramatically increased. A typical HDD may have 10–16 g of magnets,16, 17 corresponding to about 4 g of REEs, which is a fraction of a percent of the HDD mass. Large-volume HDD sources such as data centers or data destruction companies hired to dispose of legacy equipment tend to shred the drives for data security. If the shredded material can be collected in a separate waste stream, magnetic separation can markedly improve the weight fraction of REEs while other high-value metals (e.g., Au, Pd, In) will be concentrated in the non-magnetic stream. This has important implications for the economics of recycling. Nguyen et al. (2017) examined expected market conditions between 2017 and 2027 and projected that recovered REEs from US HDDs could meet up to 5.2% of neodymium permanent magnet demand outside of China, but in order for the REE recovery to be profitable, other metals (e.g., Al, Au, Cu, Ag, Pd) would also need to be recovered from HDDs.18
There are already excellent reviews of REE recycling in the literature, covering the status of the industry as well as research developments in recycling, in particular for magnets; some are provided in the references listed here and references cited therein.9, 11, 19, 20 They discuss the potential for direct magnet to magnet recycling, as well as recovery of individual REEs for reuse in magnets or other materials. For the latter, hydrometallurgical and pyrometallurgical methods are the main recovery routes. In this contribution, we briefly present some examples of newer hydrometallurgical approaches developed for REE recovery, with particular attention to advances developed within the US Department of Energy (DOE) Critical Materials Institute (CMI), an Energy Innovation Hub supported by the DOE Office of Energy Efficiency & Renewable Energy’s Advanced Manufacturing Office. Two general steps in the REE processing chain are covered, namely extraction (or leaching) and separations, but it is important to note that these steps are integrated, and indeed selective extraction methods that can advance more than one step (“process intensification”) are of particular value. Where available, the results of formal techno-economic assessment (TEA) and/or life cycle assessment (LCA) are cited.
Leaching
The most commonly reported approach for extraction of REEs into the aqueous phase is via use of mineral acids; a number of researchers has reported the leaching of REEs from permanent magnets, fluorescent phosphors, catalysts, and other REE-containing wastes by acids such as sulfuric, hydrochloric, and nitric acids.9, 21,22,23 Lister and colleagues used leaching with mineral acids (sulfuric or hydrochloric) to extract magnet REEs from the ferromagnetic fraction of e-wastes.24, 25 Starting with mixed scrap, they coupled the recovery of the REEs from the ferromagnetic fraction with processing of the non-magnetic fraction to recover base metals as well as precious metals (Figure 1), in order to make REE recovery economically viable.18 The base metals (Cu, Sn, Zn, Pb) were recovered selectively by a novel electrochemical approach that included an extraction column that enabled their separation, and the precious metals (Ag, Au, Pd) were isolated from the residues remaining after electrochemical leaching.18, 24 In addition to recovering a greater fraction of the total metal content, the approach also offered improved environmental performance, due in large part to reduced chemical and energy (electricity) consumption compared to existing pyrometallurgical and hydrometallurgical alternatives.26
As an alternative to mineral acids, some researchers have proposed the use of biologically generated leaching agents. These avoid many of the environmental hazards associated with the corrosive chemicals used in conventional hydrometallurgy, as well as the costs related to the high energy, reagent, and capital requirements.27,28,29,30 Bioleaching takes advantage of natural microbial activities, such as production of organic acids or production of sulfuric acid, with opportunities to improve on native capabilities through synthetic biology.27 Bioleaching has long been practiced for commercial recovery of some metals, in particular gold and copper,31 but recently researchers have been evaluating its efficacy for REE recovery, using both bacteria and fungi. A number of REE-containing ores and mine wastes have been subjected to bioleaching in the laboratory, including monazite,32,33,34 bastnäsite,35 and ion-adsorption clay,36 but EOL industrial or consumer products have also been tested as feedstocks for REE bioleaching. Most often biologically produced organic acids are used, because of the absence of reduced sulfur and iron content in the REE feedstocks that would otherwise make biogenic sulfuric acid generation attractive. Reed and co-workers applied the well-known gluconic acid producing bacterium Gluconobacter oxydans to recover REEs from fluorescent phosphor wastes as well as spent petroleum fluid catalytic cracking (FCC) catalyst.37, 38 Ferreira and co-workers also reported leaching from FCC catalysts, using yeasts that converted glycerol to citric acid.39 A combination of yeasts and bacteria, in a Kombucha culture fed tea and sucrose, was used by Hopfe et al. to leach REEs from phosphor wastes.40 The fact that organic acids can be produced from a variety of substrates provides versatility and the opportunity to produce leaching agents at low cost. Reed et al. took advantage of this by switching from the glucose originally used to feed G. oxydans to agricultural wastes; they found that this change resulted in the production of an organic acid mixture that was just as effective for REE leaching, but with environmental benefits and lower estimated costs.41 Such attributes could offset the slower extraction rates associated with organic acid leaching as compared with mineral acids and allow bioleaching to be used profitably on secondary sources with dilute REE content.41
Avoidance of acids altogether for leaching has also been demonstrated. Acid-free recovery of REEs from shredded e-waste, such as HDDs, or magnet swarfs is possible using copper salt solutions.42 This is an example of a technology that is suitable for magnet alloys, but would not be applied to primary rare earth ores. The NdFeB magnets (or SmCo magnets) undergo oxidative dissolution at room temperature, and the copper is precipitated and filtered out for recycling. The solution of REE3+ and Fe3+ is then mixed with ammonium oxalate to precipitate REE-oxalate, which can be calcined to produce RE2O3 while the iron can be recovered with standard approaches. The fact that this selective leaching approach can be applied to shredded HDDs, without the energy-intensive steps of pre-separation, pre-oxidation, or demagnetization and with no toxic waste generation is particularly notable. The approach has been demonstrated at the laboratory scale to recover >98% of REE content from NdFeB magnet swarf and >90% from as-shredded HDDs, and the recovered REE is suitable for reuse in magnets (Figure 2).42, 43 For a hypothetical facility processing 125 metric tons of HDDs/yr for 20 years, the net present value was estimated at $1.2 million and the internal rate of return was 38 percent.44 Recent LCA estimated that the global warming impact for producing rare earth oxides via this route could be as much as 73% lower than for prevailing rare earth production routes in China.45
Acid-free recycling of magnet waste to make new magnets.42
Another approach avoiding the use of acids, and using only water and sodium chloride, has also been reported for recovery of REE from NdFeB permanent magnets.16 In this method, the magnets are heated at 250°C in water with NaCl to remove the Ni coating on the magnets and oxidize the REE (mostly Nd) and Fe components to REE hydroxides and Fe oxides. The two powders can then be separated by magnetic separation. The metal portion of the magnetic fraction was 95% Nd, Dy, and Pr; the main impurity was Fe.16 As the water can be reused, and the NaCl is dilute (<0.5 g/L), this appears to be a promising green chemistry approach, although formal LCA or TEA for the method has not been published as yet.
Leaching of rare earths has been explored with other solvent media, including supercritical fluids.46, 47 Rare-earth salts and oxides are only sparingly soluble in supercritical fluids; however, the addition of novel adducts, such as tri-n-butyl phosphate–nitric acid (TBP–HNO3), to supercritical carbon dioxide or tetrabutyl diglycolamide in 1-octanol modified carbon dioxide can dramatically improve solubility. Extraction of REE from NdFeB magnets and lighting phosphor material using these systems has been reported.46 Again, formal published LCA or TEA is not available for this approach.
Separations
Whether for hydrometallurgical recovery from primary or secondary sources, once the REEs and other co-solubilized constituents are extracted into the aqueous phase, the next tasks are separation of REEs from non-REEs, and then further separation of individual REEs. The latter can be especially difficult because of the chemical similarity of the lanthanides.48 Early work on lanthanide separations focused on selective crystallization and precipitation,49 but solvent extraction (SX) is now the dominant commercial process for primary production of REEs, and the only proven technology for processing large volumes of REE-laden solutions.49, 50 However, hundreds of individual contactors (mixer-settlers) may be required for adequate separations,49 and consequently, high volumes of chemicals and large environmental impacts are associated with SX.51, 52 This has led to interest in the development of alternatives. These new techniques may be useful for separations of REEs from recycling feedstocks in particular, because of the differences in absolute and relative abundances and chemical speciation of the rare earths, as well as of co-occurring metals, in recycling feedstocks as compared to rare earth ores. In general, recycling feedstocks should contain a more limited number of rare earths, compared to the ores, because the original devices or products were manufactured to contain only certain REEs. This can significantly simplify the separation requirements. Also, these technologies can be applied on a smaller scale, which is more suitable for recycling as compared to primary production.
One innovative approach is the use of biosorption. The use of a column of immobilized lanmodulin, a bacterial protein shown to have extremely high affinity for lanthanides,53,54,55 was recently reported for effective separation of light lanthanides (Nd and Pr) from the higher-value heavy rare-earth dysprosium, in a simulated permanent magnet leachate.56 If non-REEs are present in the influent, they pass through the lanmodulin column, while the REEs are retained by selective binding to the lanmodulin (Figure 3). Then because the lanmodulin has higher affinity for light REEs (LREEs) than for heavy REEs (HREEs), the LREEs can be separated from HREEs by sequential stripping at different pH or citrate concentrations.56 The deployment of a natural protein for separation rather than synthetic organic ligands and organic solvents is appealing because the protein is inherently biodegradable, but the economic competitiveness of this approach will hinge in part on the stability of lanmodulin during prolonged use under realistic industrial conditions. Studies of the robustness of the protein are ongoing, as are preliminary techno-economic analyses.
Immobilized lanmodulin for REE separations.56
Another separation technology that takes advantage of simpler permanent magnet leachate compositions as compared with REE ore leachates is the recently reported CSEREOX (“chemical separation of rare-earth element oxalates”) method. In this approach, light and heavy rare earths can be separated due to their different solubilities when reacted with oxalate and an organic base. The LREEs are much less soluble and can be precipitated out first.57 The method is simple and operates at neutral pH and is expected to be more environmentally friendly and less costly compared to other state-of-the-art separation approaches that require organic solvents and or mineral acids.57 Formal TEA and LCA are in progress.
A third example of a new approach for REE separation is membrane solvent extraction (MSX), which operates on the same principles as conventional SX, but the organic solvent and extractant are immobilized within the pores of a hollow fiber filter module. The aqueous feed solution and strip solution flow through the lumen and outside of the fibers, respectively.58 The extraction and stripping (back-extraction) occur simultaneously, without equilibrium limitations, and the process can operate continuously at ambient conditions and low pressure. Recoveries of >95% and purities >99.5 wt% for Dy, Pr, and Nd were derived from magnets obtained from cell phones, hard disk drives, MRI scrap, magnet swarf, and vehicles.59 As this process has already been licensed, TEA and LCA analyses performed to date are proprietary, but the low chemical usage and energy requirements, modular design that facilitates scale-up, and low operating costs are advantages compared to traditional SX.
Conclusions and future directions
Increasing demand for more electric and greener energy systems will drive significantly higher demand for rare-earth elements, particularly those elements most closely associated with permanent magnets: Pr, Nd, Tb, and Dy. While additional primary production will be necessary to meet these needs, robust recycling of rare earth elements can be a significant source of supply in the medium term. Recycling offers several potential advantages over primary production, particularly because recycling can avoid the costly steps of separating out the low-value REEs (La and Ce) as well as co-occurring radioactive elements, and EOL products often have greater high-value REE content than many other unconventional sources (such as coal byproducts or phosphate mining wastes). Due to the distinct chemical compositions of e-waste streams, beneficiation approaches for concentrating and recovering REEs will differ from traditional REE extraction and will be economically competitive if they are compatible with recovery of other high-value materials present in e-waste such as gold, silver, platinum group metals, cobalt, and indium, while also being environmentally responsible. Innovative chemical separations paired with targeted waste streams may provide a significant source of high-value REEs to enable a more circular economy. These processes must be robust and able to respond rapidly to changes in recycling feedstock streams as product designs evolve in dynamic technology sectors. Continued long-term scientific investigation and engineering coupled with increasingly rigorous techno-economic and life cycle assessment, as well as commercial engagement, are needed to enable assessment of which recycling technologies are most economically viable and thus likely to be adopted. No one solution will be appropriate for all situations and feedstocks, and a portfolio of options will be needed to provide the agility required to support resilient supply chains for REEs.
References
International Energy Agency (IEA), The Role of Critical Minerals in Clean Energy Transitions, World Energy Outlook Special Report (IEA, Paris, France, 2021)
US Geological Survey (USGS), Mineral Commodity Summaries (USGS, Reston, VA, (2021)
S&P Global Platts, US EV market sales to rise to 6.9 million units by 2025: Frost & Sullivan (2020)
T. Fishman, T. Graedel, Nat. Sustain. 2, 332 (2019)
US Department of Energy (DOE), Wind Vision: A New Era for Wind Power in the United States (DOE/GO-102015-4557, DOE, Washington, DC, 2015). https://www.energy.gov/eere/wind/maps
European Commission, Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability (European Commission, Brussels, Belgium, 2020)
H. Hatayama, K. Tahara, Mater. Trans. 56, 229 (2015)
K. Binnemans, P. McGuiness, P.T. Jones, Nat. Rev. Mater. 6, 459 (2021)
K. Binnemans, P.T. Jones, B. Blanpain, T. Van Gerven, Y.X. Yang, A. Walton, M. Buchert, J. Clean. Prod. 51, 1 (2013)
R. Eggert, C. Wadia, C. Anderson, D. Bauer, F. Fields, L. Meinert, P. Taylor, Ann. Rev. Environ. Resour. 41, 199 (2016)
S.M. Jowitt, T.T. Werner, Z. Weng, G.M. Mudd, Curr. Opin. Green Sustain. Chem. 13, 1 (2018)
G. Gaustad, E. Williams, A. Leader, Resour. Conserv. Recycl. 167, 105213 (2021)
K. Binnemans, P.T. Jones, J. Sustain. Metall. 1, 29 (2015)
K. Binnemans, P.T. Jones, K. Van Acker, B. Blanpain, B. Mishra, D. Apelian, JOM 65, 846 (2013)
B.S. Van Gosen, P.L. Verplanck, R.R. Seal II, K.R. Long, J. Gambogi, Rare-Earth Elements (Professional Paper 1802-O), in Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply, ed. By K.J. Schulz, J.H. DeYoung Jr., R.R. Seal II, D.C. Bradley (US Geological Survey, Reston, VA, 2017), chap. O, p. 44
N. Maât, V. Nachbaur, R. Lardé, J. Juraszek, J.-M. Le Breton, ACS Sustain. Chem. Eng. 4, 6455 (2016)
I. Nlebedim, A.H. King, JOM 70, 115 (2018)
R.T. Nguyen, L.A. Diaz, D.D. Imholte, T.E. Lister, JOM 69, 1546 (2017)
Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven, P.T. Jones, J. Sustain. Metall. 3, 122 (2017)
R. Ganguli, D.R. Cook, MRS Energy Sustain. 5, E9 (2018)
C. Tunsu, M. Petranikova, M. Gergorić, C. Ekberg, T. Retegan, Hydrometallurgy 156, 239 (2015)
R.K. Jyothi, T. Thenepalli, J.W. Ahn, P.K. Parhi, K.W. Chung, J.-Y. Lee, J. Clean. Prod. 267, 122048 (2020)
M.K. Jha, A. Kumari, R. Panda, J. Rajesh Kumar, K. Yoo, J.Y. Lee, Hydrometallurgy 161, 77 (2016)
L.A. Diaz, T.E. Lister, J.A. Parkman, G.G. Clark, J. Clean. Prod. 125, 236 (2016)
T.E. Lister, M. Meagher, M.L. Strauss, L.A. Diaz, H.W. Rollins, G. Das, M.M. Lencka, A. Anderko, R.E. Riman, A. Navrotsky, Recovery of Rare Earth Elements from Recycled Hard Disk Drive Mixed Steel and Magnet Scrap, in Rare Metal Technology 2021, The Minerals, Metals & Materials Series, ed. By G. Azimi, T. Ouchi, K. Forsberg, H. Kim, S. Alam, A.A. Baba, N.R. Neelameggham (Springer, Berlin, Germany, 2021), p. 139
Z. Li, L.A. Diaz, Z. Yang, H. Jin, T.E. Lister, E. Vahidi, F. Zhao, Resour. Conserv. Recycl. 149, 20 (2019)
O. Adesina, I.A. Anzai, J.L. Avalos, B. Barstow, Chemistry 2, 20 (2017)
K. Pollmann, S. Kutschke, S. Matys, S. Kostudis, S. Hopfe, J. Raff, Minerals 6, 54 (2016)
P. Rasoulnia, R. Barthen, A.-M. Lakaniemi, Crit. Rev. Environ. Sci. Technol. 51(4), 378 (2020)
W.-Q. Zhuang, J.P. Fitts, C.M. Ajo-Franklin, S. Maes, L. Alvarez-Cohen, T. Hennebel, Curr. Opin. Biotechnol. 33, 327 (2015)
K. Bosecker, FEMS Microbiol. Rev. 20, 591 (1997)
V.L. Brisson, W.Q. Zhuang, L. Alvarez-Cohen, Biotechnol. Bioeng. 113, 339 (2015)
M.K. Corbett, J.J. Eksteen, X.-Z. Niu, J.-P. Croue, E.L.J. Watkin, Bioprocess Biosyst. Eng. 40(6), 929 (2017)
D. Shin, J. Kim, B.-S. Kim, J. Jeong, J.-C. Lee, Minerals 5, 189 (2015)
L. Zhang, H. Dong, Y. Liu, L. Bian, X. Wang, Z. Zhou, Y. Huang, Chem. Geol. 483, 544 (2018)
M.J. Barnett, B. Palumbo-Roe, S.P. Gregory, Minerals 8(6), 236 (2018)
D.W. Reed, Y. Fujita, D.L. Daubaras, Y. Jiao, V.S. Thompson, Hydrometallurgy 166, 34 (2016)
V.S. Thompson, M. Gupta, H. Jin, E. Vahidi, M. Yim, M.A. Jindra, V. Nguyen, Y. Fujita, J.W. Sutherland, Y. Jiao, D.W. Reed, ACS Sustain. Chem. Eng. 6, 1602 (2018)
D.M. Ferreira, E.F.C. Servulo, J.A.S. Silva, J. Dognini, F.J.S. Oliveira, Bioleaching of Rare Earth Elements (REE) from Spent Cracking Catalyst, in Proceedings CRETE 2018, Sixth International Conference on Industrial & Hazardous Waste Management (Chania, Crete, Greece, 2018)
S. Hopfe, K. Flemming, F. Lehmann, R. Möckel, S. Kutschke, K. Pollmann, Waste Manag. 62, 211 (2017)
H. Jin, D.W. Reed, V.S. Thompson, Y. Fujita, Y. Jiao, M. Crain-Zamora, J. Fisher, K. Scalzone, M. Griffel, D. Hartley, J.W. Sutherland, ACS Sustain. Chem. Eng. 7, 15311 (2019)
D. Prodius, K. Gandha, A.-V. Mudring, I.C. Nlebedim, ACS Sustain. Chem. Eng. 8, 1455 (2019)
REECCycling: Reclaim E-Waste (2021). http://reeccycling.com/reclaim-e-waste
S. Deng, D. Prodius, I.C. Nlebedim, A. Huang, Y. Yih, J.W. Sutherland, Sustain. Prod. Consum. 27, 1718 (2021)
N.A. Chowdhury, S. Deng, H. Jin, D. Prodius, J.W. Sutherland, I.C. Nlebedim, ACS Sustain. Chem. Eng. 9(47), 15915 (2021)
M. Case, R. Fox, D. Baek, C. Wai, Metals 9, 429 (2019)
D.L. Baek, R.V. Fox, M.E. Case, L.K. Sinclair, A.B. Schmidt, P.R. McIlwain, B.J. Mincher, C.M. Wai, Ind. Eng. Chem. Res. 55, 7154 (2016)
C.K. Gupta, N. Krishnamurthy, Int. Mater. Rev. 37, 197 (1992)
T. Cheisson, E.J. Schelter, Science 363, 489 (2019)
V. Balaram, Geosci. Front. 10, 1285 (2019)
E.O. Opare, E. Struhs, A. Mirkouei, Renew. Sustain. Energy Rev. 143, 110917 (2021)
E. Vahidi, F. Zhao, J. Environ. Manag. 203, 255 (2017)
G.J.-P. Deblonde, J.A. Mattocks, D.M. Park, D.W. Reed, J.A. Cotruvo Jr., Y. Jiao, Inorg. Chem. 59, 11855 (2020)
J.A. Cotruvo, E.R. Featherston, J.A. Mattocks, J.V. Ho, T.N. Laremore, J. Am. Chem. Soc. 140, 15056 (2018)
J.A. Mattocks, J.A. Cotruvo, Chem. Soc. Rev. (2020)
Z. Dong, J.A. Mattocks, G.J.-P. Deblonde, D. Hu, Y. Jiao, J.A. Cotruvo Jr., D.M. Park, ACS Cent. Sci. 7(11), 1798 (2021)
D. Prodius, M. Klocke, V. Smetana, T. Alammar, M.P. Garcia, T.L. Windus, I.C. Nlebedim, A.-V. Mudring, Chem. Commun. 56, 11386 (2020)
D. Kim, L.E. Powell, L.H. Delmau, E.S. Peterson, J. Herchenroeder, R.R. Bhave, Environ. Sci. Technol. 49(16), 9452 (2015)
V. Deshmane, S. Islam, R.R. Bhave, Environ. Sci. Technol. 54(1), 550 (2020)
Acknowledgments
We thank D. Combs (INL) for preparing the graphical abstract and multiple CMI colleagues for their helpful inputs to this article. This research was sponsored by the Critical Materials Institute, an Energy Innovation Hub funded by the US Department of Energy (DOE), Office of Energy Efficiency & Renewable Energy and Advanced Manufacturing Office, which supports early-stage research to advance innovation in US manufacturing and promote American economic growth and energy security. Work was performed at INL and LLNL under Contract Nos. DE-AC07-05ID14517 and DE-AC52-07NA27344, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujita, Y., McCall, S.K. & Ginosar, D. Recycling rare earths: Perspectives and recent advances. MRS Bulletin 47, 283–288 (2022). https://doi.org/10.1557/s43577-022-00301-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-022-00301-w