Abstract
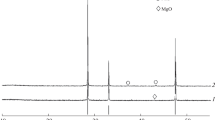
Ce0.35Zr0.65−xRExO2 (RE = Y and La; x = 0 and 0.10) and Ce0.35Zr0.50Y0.075La0.075O2 were prepared by a coprecipitation method. The textures, structures, oxygen storage capacity (OSC), and redox properties of all samples were investigated using Brunauer–Emmett–Teller surface area characterization, x-ray diffraction (XRD), Raman spectra, temperature-programmed technique, and oxygen pulse reaction. The results showed that the fresh Ce0.35Zr0.65O2 has cubic phase, 434 μmol/g of OSC, 82 m2/g of surface area, and good redox properties; after aging at 1000 °C, Ce0.35Zr0.65O2 still has cubic phase, 418 μmol/g of OSC, and 50 m2/g of surface area; when Y3+ or La3+ is added to CeO2–ZrO2, the aged Ce0.35Zr0.65−xRExO2 (RE = Y and La; x = 0 and 0.10) still remains cubic phase, high OSC, and large surface area (47 m2/g); when Y3+ and La3+ are simultaneously added into CeO2–ZrO2, a stable solid solution with cubic phase is formed and has 459 μmol/g of OSC; and the aged Ce0.35Zr0.50Y0.075La0.075O2 reaches to 60 m2/g of surface area and has 390 μmol/g of OSC.







Similar content being viewed by others
References
H.S. Gandhi and M. Shelef: The role of research in the development of new generation automotive catalysts. Stud. Surf. Sci. Catal. 30, 199–214 (1987).
G. Kim: Ceria-promoted three-way catalysts for auto exhaust emission control. Ind. Eng. Chem. Prod. Res. Dev. 21, 267–274 (1982).
M. Pijolat, M. Prin, M. Soustelle, O. Touret, and P. Nortier: Thermal stability of doped ceria: Experiment and modeling. J. Chem. Soc., Faraday Trans. 91, 3941–3948 (1995).
P. Fornasiero, R. Di Monte, G. Ranga Rao, J. Kašpar, S. Meriani, A. Trovarelli, and M. Graziani: Rh-loaded CeO2-ZrO2 solid-solutions as highly efficient oxygen exchangers: Dependence of the reduction behavior and the oxygen storage capacity on the structural-properties. J. Catal. 151, 168–177 (1995).
T. Murota, T. Hasegawa, S. Aozasa, H. Matsui, and M. Motoyama: Production method of cerium oxide with high storage capacity of oxygen and its mechanism. J. Alloys Compd. 193, 298–299 (1993).
S. Rossignol, Y. Madier, and D. Duprez: Preparation of zirconia-ceria materials by soft chemistry. Catal. Today 50, 261–270 (1999).
J. Kašpar, R. Di Monte, P. Fornasiero, M. Graziani, H. Bradshaw, and C. Norman: Dependency of the oxygen storage capacity in zirconia–ceria solid solutions upon textural properties. Top. Catal. 16-17, 83–87 (2001).
G. Balducci, J. Kašpar, P. Fornasiero, M. Graziani, M.S. Islam, and J.D. Gale: Computer simulation studies of bulk reduction and oxygen migration in CeO2−ZrO2 solid solutions. J. Phys. Chem. B 101, 1750–1753 (1997).
G. Balducci, J. Kašpar, P. Fornasiero, M. Graziani, and M.S. Islam: Surface and reduction energetics of the CeO2−ZrO2 catalysts. J. Phys. Chem. B 102, 557–561 (1998).
L.N. Ikryyannikova, A.A. Aksenov, G.L. Markaryan, G.P. Muravieva, B.G. Kostyuk, A.N. Kharlanov, and E.V. Lunina: The red-ox treatments influence on the structure and properties of M2O3-CeO2-ZrO2 (M = Y, La) solid solutions. Appl. Catal., A 210, 225–235 (2010).
H. He, H.X. Dai, and C.T. Au: Defective structure, oxygen mobility, oxygen storage capacity, and redox properties of RE-based (RE = Ce, Pr) solid solutions. Catal. Today 90, 245–254 (2004).
H. He, H.X. Dai, L.H. Ng, K.W. Wong, and C.T. Au: Pd-, Pt-, and Rh-loaded Ce0.6Zr0.35Y0.05O2 three-way catalysts: An investigation on performance and redox properties. J. Catal. 206, 1–13 (2002).
P. Vidmar, P. Fornasiero, J. Kaspar, G. Gubitosa, and M. Graziani: Effects of trivalent dopants on the redox properties of Ce0.6Zr0.4O2 mixed oxide. J. Catal. 171, 160–168 (1997).
J.X. Guo, D.D. Wu, L. Zhang, M.C. Gong, M. Zhao, and Y.Q. Chen: Preparation of nanometric CeO2-ZrO2-Nd2O3 solid solution and its catalytic performances. J. Alloys Compd. 460, 485–490 (2008).
J.X. Guo, S.H. Yuan, M.C. Gong, M. Shen, J.B. Zhong, and Y.Q. Chen: Influence of Ce0.35Zr0.55Y0.10 solid solution on performance of Pt-Rh three-way catalysts. J. Rare Earths 25, 179–183 (2007).
J.X. Guo, S.H. Yuan, M.C. Gong, L. Zhang, D.D. Wu, M. Zhao, and Y.Q. Chen: Influence of Ce0.35Zr0.55La0.10O1.95 solid solution on the performance of Pt-Rh three-way catalysts. Acta Phys. Chim. Sin. 23, 73–78 (2007).
G.A. Turko, A.S. Ivanva, L.M. Plyasova, G.S. Litvak, and V.A. Rogov: Synthesis and characterization of fluorite-like Ce-Zr-Y-La-O systems. Kinet. Catal. 46, 884–890 (2005).
J.R. McBride, K.C. Hass, B.D. Poindexter, and W.H. Weber: Raman and X-ray studies of Ce1−xRExO2−y, where RE=La, Pr, Nd, Eu, Gd and Tb. J. Appl. Phys. 76, 2435–2441 (1994).
H.C. Yao and Y.F.Y. Yao: Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 86, 254–265 (1984).
H. Zhu, Z. Qin, W. Shan, W. Shen, and J. Wang: Pd/CeO2–TiO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 225, 267–277 (2004).
M. Teng, L. Luo, and X. Yang: Synthesis of mesoporous Ce1-xZrxO2 (x = 0.2-0.5) and catalytic properties of CuO based catalysts. Microporous Mesoporous Mater. 119, 158–164 (2009).
H. He, H.X. Dai, K.W. Wong, and C.T. Au: RE0.6Zr0.4−xYxO2 (RE = Ce, Pr; x = 0, 0.05) solid solutions: An investigation on defective structure, oxygen mobility, oxygen storage capacity, and redox properties. Appl. Catal., A 251, 61–74 (2003).
C. Li, G. Zhou, L. Wang, S. Dong, J. Li, and T. Cheng: Effect of ceria on the MgO-γ-Al2O3 supported CeO2/CuCl2/KCl catalysts for ethane oxychlorination. Appl. Catal., A 400, 104–110 (2011).
Y. Teraoka, M. Yoshimatsu, N. Yamazoe, and T. Seiyama: Oxygen-sorptive properties and defect structure of perovskite-type oxides. Chem. Lett. 13, 893–896 (1984).
H.M. Zhang, Y. Shimizu, Y. Teraoka, N. Miura, and N. Yamazoe: Oxygen sorption and catalytic properties of La1−xSrxCo1−yFeyO3 perovskite-type oxides. J. Catal. 121, 432–440 (1990).
L. Xue, C. Zhang, H. He, and Y. Teraoka: Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal., B 75, 167–174 (2007).
H. Vidal, J. Kašpar, M. Pijolat, G. Colonb, S. Bernal, A. Cordón, V. Perrichon, and F. Fally: Redox behavior of CeO2–ZrO2 mixed oxides: I. Influence of redox treatments on high surface area catalysts. Appl. Catal., B 27, 49–63 (2000).
H.R. Aghabozorg, F. Sakhaie, M. Ramezani, and H. Aghabozorg: Doping of Sm into CeO2 nanotubes up to 50%. J. Sci. I. A. U. (JSIAU) 19, 47–52 (2009).
M. Yashima and T. Wakitak: Atomic displacement parameters and structural disorder of oxygen ions in the CexZr1−xO2 solid solutions (0.12 ≤ x ≤ 1.0): Possible factors of high catalytic activity of ceria-zirconia catalysts. Appl. Phys. Lett. 94, 171902 (2009).
M. Yashima, H. Arashi, M. Kakihana, and M. Yoshimura: Raman scattering study of cubic-tetragonal phase transition in Zr1-xCexO2 solid solution. J. Am. Ceram. Soc. 77, 1067–1071 (1994).
T. Nakatani, H. Okamoto, and R. Ota: Preparation of CeO2-ZrO2 mixed oxide powders by the coprecipitation method for the purification catalysts of automotive emission. J. Sol-Gel Sci. Technol. 26, 859–863 (2003).
J. Kašpar, P. Fornasiero, and M. Graziani: Use of CeO2-based oxides in the three-way catalysis. Catal. Today 50, 285–298 (1999).
T. Masui, T. Ozaki, K. Machida, and G. Adachi: Preparation of ceria–zirconia sub-catalysts for automotive exhaust cleaning. J. Alloys Compd. 303-304, 49–55 (2000).
G. Vlaic, R.D. Monte, P. Fornasiero, E. Fonda, J. Kašpar, and M. Graziani: Redox property–local structure relationships in the Rh-loaded CeO2–ZrO2 mixed oxides. J. Catal. 182, 378–389 (1999).
J.L. Ayastuy, A. Gurbani, M.P. González-Marcos, and M.A. Gutiérrez-Ortiz: Selective CO oxidation in H2 streams on CuO/CexZr1-xO2 catalysts: Correlation between activity and low temperature reducibility. Int. J. Hydrogen Energy 37, 1993–2006 (2012).
X. Wang, Q. Kang, and D. Li: Low-temperature catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts. Catal. Commun. 9, 2158–2162 (2008).
L. Cao, L. Pan, C. Ni, Z. Yuan, and S. Wang: Autothermal reforming of methane over Rh/Ce0.5Zr0.5O2 catalyst: Effects of the crystal structure of the supports. Fuel Process. Technol. 91, 306–312 (2010).
H. Zhang, Q. Yang, B. Zhang, and S. Lu: Raman spectroscopic investigation of lanthana-doped neodymium-yttria transparent ceramics. J. Raman Spectrosc. 42, 1384–1387 (2011).
G.L. Markaryan, L.N. Ikryannikova, G.P. Muravieva, A.O. Turakulova, B.G. Kostyuk, E.V. Lunina, V.V. Lunin, E. Zhilinskaya, and A. Aboukaïs: Red-ox properties and phase composition of CeO2-ZrO2 and Y2O3-CeO2-ZrO2 solid solutions. Colloids Surf., A 151, 435–447 (1999).
S.P. Kulyova, E.V. Lunina, V.V. Lunin, B.G. Kostyuk, G.P. Muravyova, and A.N. Kharlanov: Redox behavior of Y0.05Ce0.1Zr0.85O2 and Y0.1Ce0.1Zr0.8O2 system catalysts doped with copper(II). Chem. Mater. 13, 1491–1496 (2001).
D. Terribile, A. Trovarelli, J. Llorcha, C. de Leitenburg, and G. Dolcctti: The synthesis and characterization of mesoporous high-surface area ceria prepared using a hybrid organic/inorganic route. J. Catal. 178, 299–308 (1998).
E. Mamontov, T. Egami, A. Brezny, M. Koranne, and S. Tyagi: Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J. Phys. Chem. B 104, 11110–11116 (2000).
P. Fornasiero, T. Montini, M. Graziani, J. Kašpar, A.B. Hungria, A. Martinez-Arias, and J.C. Conesa: Effects of thermal pretreatment on the redox behaviour of Ce0.5Zr0.5O2: Isotopic and spectroscopic studies. Phys. Chem. Chem. Phys. 4, 149–159 (2002).
G. Balducci, P. Fornasiero, R. Di Monte, J. Kašpar, S. Meriani, and M. Grazini: An unusual promotion of the redox behaviour of CeO2-ZrO2 solid solutions upon sintering at high temperatures. Catal. Lett. 33, 193–200 (1995).
L.F. Liotta, M. Ousmane, G. Di Carlo, G. Pantaleo, G. Deganello, G. Marcì, L. Retailleau, and A. Giroir-Fendler: Total oxidation of propene at low temperature over Co3O4–CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal., A 347, 81–88 (2008).
C. Binet, M. Daturi, and J.C. Lavalley: IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 50, 207–225 (1999).
Acknowledgments
This project is financially supported by the National Nature Science Youth Fund of China (Grant No. 21173153). We would like to thank Professor Zhu, College of Materials Science and Engineering, Sichuan University for XRD measurements, and Dr. Wu, Analytical and Testing Center, Sichuan University for Raman spectra characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, J., Shi, Z., Wu, D. et al. A comparative study of Y3+- or/and La3+-doped CeO2–ZrO2-based solid solution. Journal of Materials Research 28, 887–896 (2013). https://doi.org/10.1557/jmr.2012.435
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.435