Abstract
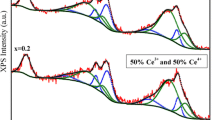
Transmission electron microscopy results from a sintered ceramics with stoichiometry of Ca(U0.5Ce0.25Hf0.25)Ti2O7 show the material contains both pyrochlore and zirconolite phases and structural intergrowth of zirconolite lamellae within pyrochlore. (001) plane of zirconolite is parallel to (111) plane of pyrochlore because of their structural similarities. The pyrochlore is relatively rich in U, Ce, and Ca with respect to the coexisting zirconolite. Average compositions for the coexisting pyrochlore and zirconolite at 1350 °C are Ca1.01(Ce3+ 0.13Ce4+ 0.19U0.52Hf0.18)(Ti1.95Hf0.05)O7 (with U/(U+Hf) = 0.72) and (Ca0.91Ce0.09)(Ce3+ 0.08U0.26Hf0.66Ti0.01)Ti2.00O7 (with U/(U+Hf) = 0.28) respectively. A single pyrochlore (Ca(U,Hf)Ti2O7) phase may be synthesized at 1350 °C if the ratio of U/(U+Hf) is greater than 0.72, and a single zirconolite (Ca(Hf,U)Ti2O7) phase may be synthesized at 1350 °C if the ratio of U/(U+Hf) is less than 0.28. An amorphous leached layer that is rich in Ti and Hf forms on the surface after the ceramics has been leached in pH 4 buffered solution. The thickness of the layer ranges from 5 nm to 15 nm. The leached layer functions as a protective layer and therefore reduces the leaching rate.
Similar content being viewed by others
References
R. G. Dosch, T. J. Headley, C. J. Northrup, and P. F. Hlava, Sandia National Laboratories Report, Sandia 82–2980, 84pp (1982).
A. E. Ringwood, S. E. Kesson, K. D. Reeve, D. M. Levins, and E. J. Ramm, Synroc. In W. Lutze and R. C. Ewing eds., “Radioactive Waste Forms for the Future.” North-Holland, Amsterdam, pp. 233–334 (1988).
A. Jostsons, E. R. Vance, D. J. Mercer, V. M. Oversby, In T. Murakami and R. C. Ewing eds. “Scientific Basis for Nuclear Waste Management XVIII.” Materials Research Society, Pittsburgh, 18 (1995), 775.
W. J. Weber, R. C. Ewing, and W. Lutze, In W. M. Murphy and D. A. Knecht eds., “Scientific Basis for Nuclear Waste Management XIX.” Materials Research Society, Pittsburgh, 19 (1996), 25.
A. J. Bakel, E. C. Buck, and B. Ebbinghaus, (1997) In “Plutonium Future — The Science.” Los Alamos National Laboratories, 135–136 (1997).
B. D. Begg, and E. R. Vance, In W. J. Gray and I. R. Triay eds. “Scientific Basis for Nuclear Waste Management XX.” Materials Research Society, Pittsburgh, 20 (1997), 333.
B. D. Begg, E. R. Vance, R. A. Day, M. Hambley, and S. D. Conradson In W. J. Gray and I. R. Triay eds. “Scientific Basis for Nuclear Waste Management XX.” Materials Research Society, Pittsburgh, 20 (1997), 325.
E. C. Buck, B. Ebbinghaus, A. J. Bakel, and J. K. Bates, In W. J. Gray and I. R. Triay eds. “Scientific Basis for Nuclear Waste Management XX.” Materials Research Society, Pittsburgh, 20 (1997), 1259.
E. R. Vance, MRS Bulletin, 19 (1994), 28.
E. R. Vance, A. Jostsons, M. W. A. Stewart, R. A. Day, B. D. Begg, M. J. Hambley, K. P. Hart, and B. B. Ebbinghaus, In “Plutonium Future — The Science.” Los Alamos National Laboratories, page 19 (1997).
E. R. Vance, K. P. Hart, R. A. Day, M. L. Carter, M. Hambley, M. G. Blackford, and B. D. Begg, In W. J. Gray and I. R. Triay eds. “Scientific Basis for Nuclear Waste Management XX.” Materials Research Society, Pittsburgh, 20 (1997), 341.
H. Xu, and Y. Wang, J. of Nuclear Materials, 275 (1999), 216.
Y. Wang, and H. Xu, In R. W. Smithe and D. W. Shoesmith ed. “Scientific Basis for Nuclear Waste Management XXIII.” Materials Research Society, Pittsburgh, 23 (2000), 367.
G. R. Lumpkin, K. L. Smith, G. Mark, and M. G. Blackford, In T. Murakami and R. C. Ewing eds. “Scientific Basis for Nuclear Waste Management XVIII.” Materials Research Society, Pittsburgh, 18 (1995), 885.
A. G. Solomah, T. S. Sridhar, and S. C. Jones, In “Advances in Ceramics, Vol. 20, Nuclear Waste Management II, American Ceramic Society, Columbus, p. 259 (1996).
L. L. Hench, D. E. Clarke, and J. Campbell, Chemical Waste Management, 5 (1984), 149.
H. Xu, and Y. Wang, J. of Nuclear Materials, 279 (2000), 100.
J. A. Fortner, E. C. Buck, A. J. G. Ellison, and J. K. Bates, Ultramicroscopy, 67 (1997), 77.
H. Xu, Y. Wang, and L. L. Barton, J. of Nuclear Materials, 265 (1999), 117.
R. Giré, R. J. Swope, E. C. Buck, R. Guggenheim, D. Mathys, and E. Reusser, In Robert W. Smith and David W. Shoesmith eds. “Scientific Basis for Nuclear Waste Management XXIII.” Materials Research Society, Pittsburgh, 23 (2000), 519.
T. J. White, American Mineralogist, 69 (1984), 1156.
P. Bayliss, F. Mazzi, R. Munno, T. J. White, Mineralogical Magazine, 53 (1989), 565.
H. Xu, Y. Wang, and L. L. Barton, J. of Nuclear Materials, 273 (1999), 343.
K. G. Knauss, W. L. Bourcier, K. D. McKeegan, C. I. Merzbacher, S. N. Nguyen, F. J. Ryerson, D. K. Smith, H. C. Weed, and L. Newton, Mat. Res. Soc. Symp. Proc.176 (1990), 371.
S. K. Roberts, W. L. Bourcier, and H. F. Shaw, Radiochim. Acta., 88 (2000), 539.
K. B. Helean, A. Navrotsky, E. R. Vance, M L. Carter, B. Ebbinghaus, O. Krikorian, J. Lian, L. M. Wang, and J. G. Catalano, J. Nuclear Materials, 303 (2002), 226.
J. A. Fortner, A. J. Fropf, R. J. Finch, A. J. Bakel, M. C. Hash, and D. B. Chamberlain, J. Nuclear Materials, 304 (2002), 56.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Wang, Y., Zhao, P. et al. U- and Hf-Bearing Pyrochlore and Zirconolite and their Leached Layers Formed in Acidic Solution: Tem Investigation. MRS Online Proceedings Library 757, 62 (2002). https://doi.org/10.1557/PROC-757-II6.2
Published:
DOI: https://doi.org/10.1557/PROC-757-II6.2