Abstract
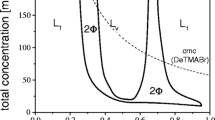
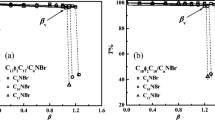
We have prepared spontaneous, single-walled, equilibrium vesicles of controlled size and surface charge from aqueous mixtures of simple, commercially available, single-tailed cationic and anionic surfactants. We believe vesicle formation results from the production of anion-cation surfactant pairs which then act as double-tailed zwitterionic surfactants. Although unilamellar vesicles have been created by numerous physical and chemical techniques from multilamellar dispersions, all such vesicle systems revert to the equilibrium, multilamellar phase over time. These catanionic vesicles are stable for periods as long as several years and appear to be the equilibrium form of aggregation. Here we review the phase behavior and structural studies of several such mixtures, with particular focus on the effect of surfactant tail lengths on size and location of the vesicle phase in the appropriate phase diagram. The approach to equilibrium is also discussed.
Similar content being viewed by others
References
A.D. Bangham, M.M. Standish, and J.C. Watkins, J. Mol. Biol. 13, 238 (1965).
J. Fendler, Membrane Mimetic Chemistry (Wiley, New York, 1983).
M. J. Ostro, Ed., Liposomes: From Biophysics to Therapeutics (Marcel Dekker, New York, 1987).
S. Bhandarkar, A. Bose, J. Colloid Interface Sci. 135, 531 (1990).
J. Zasadzinski, L.E. Scriven, and H.T. Davis, Phil Mag. A 51, 287 (1985).
Both uni- and multilamellar lipid and surfactant aggregates are called liposomes. Within this classification are three acronyms - MLV for multilamellar vesicle, SUV for small unilamellar vesicle (<100 nm diameter) and LUV for large unilamellar vesicle (>100 nm). See: D. Papahadjopolous, Ann. NY Acad. Sci. 308, 367 (1978).
F. Szoka and D. Papahadjopolous, Ann. Rev. Biophys. Bioeng. 2, 467 (1980).
J. Virdon (private communication).
L. Rydhag, P. Stenius, L. Ödberg, J. Coll. Interface Sci. 86, 275 (1982).
H. Hauser, N. Gains, H. Eibl, M. Müller, E. Wehrli, Biochemistry 25, 2126 (1986).
L. Gros, H. Ringsdorf, H. Schupp, Angew. Chem. Int. Ed. Engl. 20, 305 (1981).
J. Zasadzinski, P. Vosejpka, W.G. Miller, J. Coll. Interface Sci. 110, 347 (1986).
E.W. Kaler, K.L. Herrington, A.K. Murthy, and J. Zasadzinski, J. Phys. Chem. (in press).
E.W. Kaler, A. K. Murthy, B. Rodriguez, J. Zasadzinski, Science 245, 1371 (1989).
E.W. Kaler, K.L. Herrington, D.D. Miller, and J. Zasadzinski, in Structure and Dynamics of Supramolecular Aggregates, edited by S.H. Chen, J.S. Huang, and P. Tartaglia (Kluwer Academic Publishers, Dordrecht, 1992).
A.K. Murthy, E. W. Kaler, and J. A. N. Zasadzinski, J. Coll. Interface Sci. 145, 598 (1991).
C. Gamboa and L. Sepulveda, J. Coll. Interface Sci., 113, 566 (1986).
T. Shikata, H. Hirata, and T. Kotaka, Langmuir 3, 1081 (1987).
J. Weers, E. W. Kaler, and K. L. Herrington, manuscript in preparation.
C. Tanford, The Hydrophobic Effect (Wiley, New York, 1980).
J. Israelachvili, D. J. Mitchell and B. W. Ninham, J.Chem. Soc. Faraday Trans. II, 22, 1525 (1976).
J. Israelachvili, Intermolecular and Surface Forces (Academic Press, New York, 1985).
K. L. Herrington and E. W. Kaler, manuscript in preparation.
S. A. Safran, P. Pincus, and D. AndelmanScience 248, 354 (1990).
S. A. Safran, P. Pincus, D. Andelman, and F.C. MacKintosh, Phys. Rev. A 43, 1071 (1991).
W. Helfrich, Z. Naturforsch. 28c, 693 (1973).
W. Helfrich, Z. Naturforsch 33a, 305 (1978).
L. Cantu, M. Corti, M. Musolino, P. Salina, Europhys. Lett. 3, 561 (1990).
Acknowledgements
We acknowledge many useful discussions with Jacob Israelachvili, Fyl Pincus, Sam Safran, and Fred Mackintosh on the theoretical aspects of spontaneous vesicles. JANZ acknowledges financial support from the National Science Foundation (CBT 86-57444) and the Petroleum Research Fund, administered by the ACS, for partial support of this work. EWK acknowledges support from the University of Delaware Research Foundation, The Clorox Company, and the National Science Foundation (PYIA 83-51179 and CTS 91-02719).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaler, E.W., Herrington, K.L. & Zasadzinski, J.A.N. Spontaneous Vesicles and other Solution Structures in Catanionic Mixtures. MRS Online Proceedings Library 248, 3–10 (1991). https://doi.org/10.1557/PROC-248-3
Published:
Issue Date:
DOI: https://doi.org/10.1557/PROC-248-3