Abstract
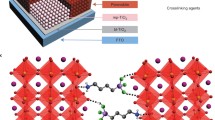
Perovskite materials are sensitive to environmental conditions. Here we report the synthesis and characterization of a hydrophobic alkylammonium lead(ll) iodide perovskite with enhanced stability in water. Water stability was achieved by growing a shell of 4-[(N-3-butyne)carbox-yamido]anilinium lead(ll) iodide over methylammonium lead(ll) iodide. As a proof of concept, the water-splitting reaction was performed using our new material coated on TiO2, and a 7-fold increase in applied bias photon-to-current efficiency was observed as compared with standard p25-TiO2. Such simple and versatile chemical modification to induce high water stability is useful toward exploring new applications for the perovskite materials.




Similar content being viewed by others
References
A. Kojima, K. Teshima, Y. Shirai, and T. Miyasaka: Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem.Soc. 131, 6050 (2009).
M.M. Lee, J. Teuscher, T. Miyasaka, T.N. Murakami, and H.J. Snaith: Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338, 643 (2012).
H. Zhou, Q. Chen, G. Li, S. Luo, T-B. Song, H-S. Duan, Z. Hong, J. You, Y. Liu, and Y. Yang: Interface engineering of highly efficient perovskite solar cells. Science 345, 542 (2014).
J. Yang, B.D. Siempelkamp, D. Liu, and T.L. Kelly: Investigation of CH3NH3Pbl3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 9, 1955 (2015).
D. Wang, M. Wright, N.K. Elumalai, and A. Uddin: Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 147, 255 (2016).
S. Kumar and A. Dhar: Accelerated thermal-aging-induced degradation of organometal triiodide perovskite on ZnO nanostructures and its effect on hybrid photovoltaic devices. ACS Appl. Mater. Interfaces 8, 18309 (2016).
Q. Jiang, D. Rebollar, J. Gong, E.L. Piacentino, C. Zheng, and T. Xu: Pseudohalide-induced moisture tolerance in perovskite CH3NH3Pb (SCN)2I thin films. Angew. Chem. Int. Ed. 54, 7617 (2015).
S. Yang, Y. Wang, P. Liu, Y.B. Cheng, H.J. Zhao, and H.G. Yang: Functionalization of perovskite thin films with moisture-tolerant molecules. Nat. Energy 1, 15016 (2016).
G. Niu, W. Li, F. Meng, L. Wang, H. Donga, and Y. Qiu: Study on the stability of CH3NH3Pbl3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2, 705 (2014).
L. Zheng, Y.H. Chung, Y. Ma, L Zhang, L. Xiao, Z. Chen, S. Wang, B. Qu, and Q. Gong: A hydrophobic hole transporting oligothiophene for planar perovskite solar cells with improved stability. Chem. Commun. 50, 11196 (2014).
P. Da, M. Cha, L. Sun, Y. Wu, Z-S. Wang, and G. Zheng: High-performance perovskite photoanode enabled by Ni passivation and catalysis. Nano Lett. 15, 3452 (2015).
J.H. Kim, Y. Jo, J.H. Kim, J.W. Jang, H.J. Kang, Y.H. Lee, D.S. Kim, Y. Jun, and J.S. Lee: Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in Tandem: a dual absorber artificial leaf. ACS Nano 9, 11820 (2015).
D.N. Dirin, S. Dreyfuss, M.I. Bodnarchuk, G. Nedelcu, P. Papagiorgis, G. Itskos, and M.V. Kovalenko: Lead halide perovskites and other metal halide complexes as inorganic capping ligands for colloidal nanocrystals. J. Am. Chem. Soc. 136, 6550 (2014).
I. Hwang, I. Jeong, J. Lee, M.J. Ko, and K. Yong: Enhancing stability of perovskite solar cells to moisture by the facile hydrophobic passivation. ACS Appl. Mater. Interfaces!, 17330 (2015).
S. Guarnera, A. Abate, W. Zhang, J.M. Foster, G. Richardson, A. Petrozza, and H.J. Snaith: Improving the long-term stability of perovskite solar cells with a porous Al2O3 buffer layer. J. Phys. Chem. Lett. 6, 432 (2015).
H-S. Kim, C-R. Lee, J-H. Im, K-B. Lee, T. Moehl, A. Marchioro, S-J. Moon, R. Humphry-Baker, J-H. Yum, J.E. Moser, M. Gratzel, and N-G. Park: Lead iodide perovskite sensitized all-solid-state submicron thin film meso-scopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012).
S. Ito, P. Chen, P. Comte, M.K. Nazeeruddin, P. Liska, P. Pechy, and M. Gratzel: Fabrication of screen-printing pastes from Ti02 powders for dye-sensitised solar cells. Prog. Photovolt: Res. Appl. 15, 603 (2007).
T. Oku: Crystal Structures of CH3NH3Pbl3 and Related Perovskite Compounds Used for Solar Cell, Solar Cells-New Approaches and Reviews, ed. L.A. Kosyachenko, InTech, Rijeka, Croatia ISBN: 78-953-51-2184-8, DOI: 10.5772/59284. (2015). Available at: http:// www.intechopen.com/books/solar-cells-new-approaches-and-reviews/crystal-structures-of-ch3nh3pbi3-and-related-perovskite-compounds-used-for-solar-cells.
F. Maxim, P. Ferreira, P.M. Vilarinho, and I. Reaney: Hydrothermal synthesis and crystal growth studies of BaTiO3 using Ti nanotube precursors. Cryst Growth Des. 8, 3309 (2008).
H. Brune, C. Romainczyk, H. Roder, and K. Kern: mechanism of the transition from fractal to dendritic growth of surface aggregates. Nature 369, 469 (1994).
B. Ranguelov, D. Goranova, V. Tonchev, and R. Yakimova: Diffusion limited aggregation with modified local rules. C R. Acad. Bulg. Sci. 65, 913 (2012).
K. Gelderman, L. Lee, and S.W. Donne: Flat-band potential of a semiconductor: W using the Mott-Schottky equation. J. Chem. Educ. 84, 685 (2007).
M. Gratzel: Photoelectrochemical cells. Nature 414, 338 (2001).
S. Aharon, A. Dymshits, A. Rotem, and L. Edgar: Temperature dependence of hole conductor free formamidinium lead iodide perovskite based solar cells. J. Mater. Chem. A 3, 9171 (2015).
T. Hisatomi, J. Kubota, and K. Domen: Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520 (2014).
R. Daghrir, P. Drogui, and D. Rober: Modified TiO2 for environmental photocatalytic applications: a review. Ind. Eng. Chem. Res. 52, 3581 (2013).
Z. Chen, T.F. Jaramillo, T.G. Deutsch, A. Kleiman-Shwarsctein, A. J. Forman, N. Gaillard, R. Garland, K. Takanabe, C. Heske, M. Sunkara, E.W. McFarland, K. Domen, E.L. Miller, J.A. Turner, and H.N. Dinh: Accelerating materials development for photoelectrochemical hydrogen production: standards for methods, definitions, and reporting protocols. J. Mater. Res. 25, 3 (2010).
A.P. Upadhyay, D.K. Behara, G.P. Sharma, M. Gyanprakash, R.G.S. Pala, and S. Sivakumar: Fabricating appropriate band-edge-staggered hetero-semiconductors with optically activated Au nanoparticles via click chemistry for photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 4, 4511 (2016).
Y. Gao, X. Ding, J. Liu, L Wang, Z. Lu, L. Li, and L. Sun: Visible light driven water splitting in a molecular device with unprecedentedly high photocurrent density. J. Am. Chem. Soc. 135, 4219 (2013).
J. Wang, H.X. Zhong, Y.L. Qin, and X.B. Zhang: An efficient three-dimensional oxygen evolution electrode. Angew. Chem. Int. Ed. 52, 5248 (2013).
K.H. Ji, D.M. Jang, Y.J. Cho, Y. Myung, H.S. Kim, Y. Kim, and J. Park: Comparative photocatalytic ability of nanocrystal-carbon nanotube and -TiO2 nanocrystal hybrid nanostructures. J. Phys. Chem. C 113, 19966 (2009).
Acknowledgments
The authors gratefully acknowledge the financial support from the Technology System Development program of the Department of Science and Technology, Government of India via project DST/TSG/SH/2011/106. S. S. and S. V. acknowledge the funding support from the National University of Singapore under the joint Ph.D. program between NUS and IITK. S. S. K. acknowledges the Centre for Environmental Science & Engineering, Thematic Unit of Excellence on Soft Nanofabrication, IITK.
Author information
Authors and Affiliations
Corresponding authors
Supplementary material
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1557/mrc.2018.56
Rights and permissions
About this article
Cite this article
Sasmal, S., Valiyaveettil, S., Upadhyay, A.P. et al. Alkyne-modified water-stable alkylammonium lead (II) iodide perovskite. MRS Communications 8, 289–296 (2018). https://doi.org/10.1557/mrc.2018.56
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2018.56