Abstract
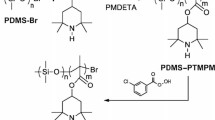
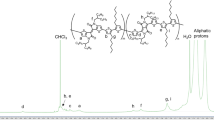
Polymers with stable radical groups are promising materials for organic electronic devices due to their unique redox activity. Block copolymers with one redox active block could be used in nanostructured devices for electronic applications. We report on the synthesis and characterization of such multifunctional block copolymers in which phase separation on the 10 nm (half pitch) scale is achieved by using fluorinated blocks. Fluorination of one block increases the degree of phase separation and leads to smaller accessible domain sizes. Block copolymers with 60%, 80% and 90% of a stable radical containing block and either fluorinated or non-fluorinated second blocks were made by atom transfer radical polymerization, and their microstructure formation as a function of fluorine content is described after solvent vapor or thermal annealing. Electrical characterization of such a partly fluorinated block copolymer shows their potential for electronic devices.






Similar content being viewed by others
References
O.H. Griffith, J.F.W. Keana, S. Rottschaefer, and T.A. Warlick: Preparation and magnetic resonance of nitroxide polymers. J. Am. Chem. Soc. 89, 5072 (1967).
K. Nakahara, S. Iwasa, M. Satoh, Y. Morioka, J. Iriyama, M. Suguro, and E. Hasegawa: Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 359, 351 (2002).
K. Oyaizu and H. Nishide: Radical polymers for organic electronic devices: a radical departure from conjugated polymers?Adv. Mater. 8555, 2339 (2009).
K. Nakahara, K. Oyaizu, and H. Nishide: Organic radical battery approaching practical use. Chem. Lett. 40, 222 (2011).
T. Janoschka, M.D. Hager, and U.S. Schubert: Powering up the future: radical polymers for battery applications. Adv. Mater. 24, 6397 (2012).
H-Q. Li, Y. Zou, and Y-Y. Xia: A study of nitroxide polyradical/activated carbon composite as the positive electrode material for electrochemical hybrid capacitor. Electrochim. Acta 52, 2153 (2007).
M. Kamachi, M. Tamaki, Y. Morishima, S-i Nozakura, W. Mori, and M. Kishita: Electron exchange phenomena of polymers containing nitroxyl radicals. Polym. J. 14, 363 (1982).
F. MacCorquodale, J.A. Crayston, J.C. Walton, and D.J. Worsfold: Synthesis and electrochemical characterization of poly(TEMPO-Acrylate). Tetrahedron Lett. 31, 771 (1990).
Y. Yonekuta, K. Susuki, K. Oyaizu, K. Honda, and H. Nishide: Battery-inspired, nonvolatile, and rewritable memory architecture: a radical polymer-based organic device. J. Am. Chem. Soc. 129, 14128 (2007).
T. Sukegawa, H. Omata, I. Masuko, K. Oyaizu, and H. Nishide: Anionic polymerization of 4-methacryloyloxy-TEMPO using an MMA- capped initiator. ACS Macro Lett. 3, 240 (2014).
K. Oyaizu, Y. Ando, H. Konishi, and H. Nishide: Nernstian adsorbate-like bulk layer of organic radical polymers for high-density charge storage purposes. J. Am. Chem. Soc. 130, 14459 (2008).
T. Suga, M. Sakata, K. Aoki, and H. Nishide: Synthesis of pendant radical- and ion-containing block copolymers via ring-opening metathesis polymerization for organic resistive memory. ACS Macro Lett. 3, 703 (2014).
L. Rostro, A.G. Baradwaj, and B.W. Boudouris: Controlled radical polymerization and quantification of solid state electrical conductivities of macromolecules bearing pendant stable radical groups. ACS Appl. Mater. Interfaces 5, 9896 (2013).
X. Zhuang, C. Xiao, K. Oyaizu, N. Chikushi, X. Chen, and H. Nishide: Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J. Polym. Sci. Part A Polym. Chem. 48, 5404 (2010).
T. Janoschka, A. Teichler, A. Krieg, M.D. Hager, and U.S. Schubert: Polymerization of free secondary amine bearing monomers by RAFT polymerization and other controlled radical techniques. J. Polym. Sci. Part A Polym. Chem. 50, 1394 (2012).
T. Uemukai, T. Hioki, and M. Ishifune: Thermoresponsive and redox behaviors of poly(N-isopropylacrylamide)-based block copolymers having TEMPO groups as their side chains. Int. J. Polym. Sci. 2013, 196145 (2013).
K. Saito, K. Hirose, T. Okayasu, H. Nishide, T. Milton, and W. Hearn: TEMPO radical polymer grafted silicas as solid state catalysts for the oxidation of alcohols. RSC Adv. 3, 9752 (2013).
Y. Wang, M. Hung, and C. Lin: Patterned nitroxide polymer brushes for thin-film cathodes in organic radical batteries. Chem. Commun. 47, 1249 (2011).
H. Lin, C. Li, and J. Lee: Nitroxide polymer brushes grafted onto silica nanoparticles as cathodes for organic radical batteries. J. Power Sources 196, 8098 (2011).
C-H. Lin, W-J. Chou, and J-T. Lee: Three-dimensionally ordered macroporous nitroxide polymer brush electrodes prepared by surface-initiated atom transfer polymerization for organic radical batteries. Macromol. Rapid Commun. 33, 107 (2012).
C. Lin, C. Chau, and J. Lee: Polymer Chemistry Synthesis and characterization of polythiophene grafted with a nitroxide radical polymer via atom transfer radical polymerization. Polym. Chem. 1467 (2012).
M-K. Hung, Y-H. Wang, C-H. Lin, H-C. Lin, and J-T. Lee: Synthesis and electrochemical behaviour of nitroxide polymer brush thin-film electrodes for organic radical batteries. J. Mater. Chem. 22, 1570 (2012).
G. Hauffman, J. Rolland, J. Bourgeois, and A. Vlad: Synthesis of nitroxide-containing block copolymers for the formation of organic cathodes. J. Polym. Sci. Part A Polym. Chem. 51, 101 (2013).
F. Behrends, H. Wagner, A. Studer, O. Niehaus, R. Pöttgen, and H. Eckert: Polynitroxides from Alkoxyamine Monomers: Structural and Kinetic Investigations by Solid State NMR. Macromolecules 46, 2553 (2013).
M.W. Matsen, and F.S. Bates: Unifying weak- and strong-segregation block copolymer theories. Macromolecules 29, 1091 (1996).
A. Nunns, J. Gwyther, and I. Manners: Inorganic block copolymer lithography. Polymer (Guildf). 54, 1269 (2013).
M.A. Hillmyer, and T.P. Lodge: Synthesis and self-assembly of fluorinated block copolymers. J. Polym. Sci. Part A Polym. Chem. 40, 1 (2002).
H. Nishide, S. Iwasa, Y-J. Pu, T. Suga, K. Nakahara, and M. Satoh: Organic radical battery: nitroxide polymers as a cathode-active material. Electrochim. Acta 50, 827 (2004).
Acknowledgments
C. L. acknowledges financial support by the Deutsche Forschungsgemeinschaft (German Research Foundation, Forschungsstipendium Li 2526/2-1). A. M. acknowledges support by a seed grant from the Cornell Center for Materials Research, an NSF MRSEC program (DMR-1120296). AFM measurements were performed at the Cornell NanoScale Facility, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation (grant ECCS-0335765). NMR spectroscopy was measured at the Cornell University NMR Facility. EPR spectroscopy was performed at ACERT, which is supported by the National Institute of General Medical Sciences of the National Institutes of Health (Award Number P41GM103521). We composed coin cells in the laboratories of Lynden A. Archer and thank Sampson Lau for assistance and help with the measurements of the charge-discharge behavior. We thank Boris Dzikovski for help with EPR measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary materials
Supplementary materials
For supplementary material for this article, please visit http://dx.doi.org/10.1557/mrc.2015.50
Rights and permissions
About this article
Cite this article
Liedel, C., Moehle, A., Fuchs, G.D. et al. Block copolymers with stable radical and fluorinated groups by ATRP. MRS Communications 5, 441–446 (2015). https://doi.org/10.1557/mrc.2015.50
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2015.50