Abstract
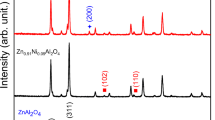
Thermal analysis and x-ray diffraction have confirmed that single-phase, cubic-spinel-structured NiMn2O4 (2:1 Mn2O3:NiO) begins to decompose into a rocksalt and a second spinel-structured phase above 907 °C. The decomposition product in samples air-quenched from 900 and 1200 °C was therefore investigated using transmission electron microscopy. Samples quenched from 900 °C (below decomposition temperature) did not show the expected single-phase microstructure, but instead grains contained nanoregions of a lenticular fringe contrast. Samples quenched from 1050 to 1200 °C were generally composed of Mn-rich spinel grains in addition to grains containing Mn-rich spinel precipitates (30–50 nm) surrounded by a Ni-rich rocksalt matrix. As temperature increased, the spinel grains and precipitates became clearly tetragonal, exhibiting a ferroelastic domain structure arising from a cooperative Jahn–Teller distortion. A decomposition mechanism based on the degree of inversion is proposed to explain these microstructures. Slow cooling samples from 1250 °C resulted in partial recomposition, leading to a microstructure principally composed of cubic spinel and regions of much smaller spinel structured precipitates (50–120 nm) in a rocksalt structured matrix. The slow-cooled samples showed a larger increase in resistance over time than single-phase samples did when held at 400 °C. X-ray diffraction measurements carried out before and after electrical characterization showed a reduction in the amount of rocksalt structured material present in slow-cooled samples.
Similar content being viewed by others
References
E.G. Larson, R.J. Arnott, and D.G. Wickham, J. Phys. Chem. Solids 23, 1771 (1962).
B. Gillot, M. Kharroubi, R. Metz, R. Legros, and A. Rousset, Solid State Ionics 44, 3–4, 275 (1991).
D.F. Shriver, P.W. Atkins, and C.H. Langford, Inorganic Chemistry (Oxford University Press, Oxford, U.K., 1992).
J.J. Couderc, S. Fritsch, M. Brieu, G. Vanderschaeve, M. Fagot, and A. Rousset, Philos. Mag. B 70, 1077 (1994).
C. Metzmacher, I.M. Reaney, and W. Groen, Phys. Status Solidi A 181, 369 (2000).
A.Ya. Fishman, M.A. Ivanov, V.Ya. Mitrofanov, and A.A. Shemyakov, Phys. Status Solidi B 160, 153 (1990).
V.A.M. Brabers and J.C.J. Terhell, Phys. Status Solidi A 6, 325 (1982).
E.J. Verwey, P.W. Haayman, F.C. Romeyn, and G.W. van Oosterhout, Philips Res. Rep. 5, 173 (1950).
S.E. Dorris and T.O. Mason, J. Am. Ceram. Soc. 71, 379 (1988).
W.A. Groen, V. Zaspalis, and S. Schuurman, J. Mater. Sci. Lett. 18(15), 1233 (1999).
B. Boucher, R. Buhl, and M. Perrin, Acta Crystallogr. B 25, 2326 (1969).
A. Feltz, J. Toepfer, and F. Schirrmeister, J. Eur. Ceram. Soc. 9, 187 (1992).
D.G. Wickham, J. Inorg. Nucl. Chem. 26, 1369 (1963).
J. Jung, J. Topfer, J. Murbe, and A. Feltz, J. Eur. Cer. Soc. 6, 351 (1990).
G.D.C. Csete de Gyorgyfalva, I.M. Reaney, C. Metzmacher, and W. Groen, Proc. EMAG 99. (Inst. Phys. Conf. Series 161, London, U.K., 1999), pp. 529–532.
G.D.C. Csete de Györgyfalva, A.N. Nolte, and I.M. Reaney, J. Eur. Ceram. Soc. 19, 857 (1999).
H. Schmalzried, Solid State Reactions, 2nd ed. (Verlag Chemie, Weinheim, Germany, 1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Györgyfalva, G.D.C.C., Reaney, I.M. Decomposition of NiMn2O4 spinels. Journal of Materials Research 18, 1301–1308 (2003). https://doi.org/10.1557/JMR.2003.0179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2003.0179