Abstract
Insulating silicon dioxide (SiO2) films can be produced by hydrolysis of metal alkoxide tetraethylorthosilicate (TEOS) in the presence of an acid catalyst in supercritical fluid CO2 (sc-CO2). In this study, SiO2 films are formed on different substrates using TEOS as a source of silicon, and acetic acid (HAc) as a catalyst. Water required for the hydrolysis reaction is from in situ generation of esterification and condensation reactions involving HAc and the alcohol produced. The acid catalyzed deposition reaction actually starts at room temperature but produces decent films in sc-CO2 at moderately high temperatures (e.g. 50 °C). Supercritical fluid CO2 is known to have near zero surface tension and provides an ideal medium for fabrication of SiO2 films. Formation of SiO2 films via hydrolysis reaction in sc-CO2 is more rapid compared to the traditional hydrolysis reaction at room temperature. In general, metal alkoxide hydrolysis reactions carried out in a closed sc-CO2 system is not affected by moisture in air compared with traditional open-air hydrolysis systems. Using sc-CO2 as a reaction medium can eliminate undesirable organic solvents utilized in traditional alkoxide hydrolysis reactions.
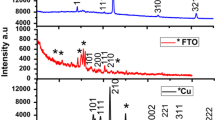
X-ray diffraction (XRD) and electron diffraction (ED) measurements demonstrated that the SiO2 films produced are amorphous. Energy dispersive spectroscopy (EDS), attenuated total reflectance-Fourier transform infrared (ATR-FTIR) and X-ray photoelectron (XPS) spectroscopy show elemental compositions of the films formed on the substrate surfaces to be SiO2. Film thickness formation by controlling the amount of the catalyst is discussed.
Similar content being viewed by others
References
A. Perucchi, L. Baldassarre, P. Postorino and S. Lupi, J. Phys. : Condensed Matter, 21, 323202 (2009).
M. Izumi, Y. Murakami, Y. Konishi, T. Manako, M. Kawasaki and Y. Tokura, Phys. Rev. B 60, 1211 (1999).
M. K. Gangishetty, R. W. J. Scott and Timothy L. Kelly, Langmuir 30, 14352 (2014).
S. Takenaka, H. Matsumori, K. Nakagawa, H. Matsune, E. Tanabe and M. Kishida, J. Phys. Chem. C 111, 15133 (2007).
H. Chen, T. Ming, S. Zhang, Z. Jin, B. Yang and J. Wang, ACS Nano 5, 4865 (2011).
M. H. Park, Y. J. Jang, H. M. Sung-Suh and M. M. Sung, Langmuir 20, 2257 (2004).
D.A. Racke, L.L. Kelly, H. Kim, P. Schulz, A. Sigdel, J.J. Berry, S. Graham, D. Nordlund and O.L.A. Monti, J. Phys. Chem. Lett. 6, 1935, (2015).
A. Hozumi, S. Kojima, S. Nagano, T. Seki, N. Shirahata and T. Kameyama, Langmuir 23, 3265 (2007).
Z. Chen, W. Li, R. Li, Y. Zhang, G. Xu and H. Cheng, Langmuir 29, 13836 (2013).
K. Xu and J. R. Heath, Nano Lett. 8, 3845 (2008).
J. S. Wang, C. M. Wai, G. J. Brown and S. D. Apt. RSC Adv. 5, 74753 (2015).
J. W. Stouwdam, J. N. Shan and F.C.J.M. van Veggel, J. Phys. Chem. C 111, 1086 ((2007).
R. Ihly, J. Tolentino, Y. Liu, M. Gibbs and M. Law, ACS Nano 5, 8175 (2011).
M. Nanu, J. Schoonman and A. Goossens, Adv. Mater. 16, 453 (2004).
A. Pourret, P.G. Sionnest and J.W. Elan, Adv. Mater. 21, 232 (2001).
A. M. Buckley and M. Greenblatt, J. Chem. Edu.,71, 599 (1994).
B. Karmakar, G. De and D. Ganguli, J. Non-Cryst Solids 272, 119 (2000).
S. S. Jada, J. Am. Ceram. Soc., 70, C-298 (1987).
A. N. Sabirzyanov, A. P. Il’in, A. P. Akhunov and F. M. Gumerov, High Temperature, 40, 203 (2002).
E. J. A. Pope and J. D. Mackenzie, J. Non-Cryst. Solids 87, 185 (1986).
P. A. Charpentier, X. S. Li and R. H. Sui, Langmuir 25, 3748 (2009).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, J.S., Wai, C.M., Brown, G.J. et al. Formation of Insulating Oxide Films with Hydrolysis Reactions of Alkoxide Precursors in Supercritical Fluid CO2: Chemistry, Morphology, Characterization and Film Thickness. MRS Advances 1, 2591–2596 (2016). https://doi.org/10.1557/adv.2016.269
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2016.269