-
PDF
- Split View
-
Views
-
Cite
Cite
Bodil Andersson, Roland Andersson, Johan Brandt, Peter Höglund, Lars Algotsson, Johan Nilsson, Gastrointestinal complications after cardiac surgery – improved risk stratification using a new scoring model, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 3, March 2010, Pages 366–370, https://doi.org/10.1510/icvts.2009.219113
Close - Share Icon Share
Abstract
Gastrointestinal (GI) complications are serious consequences of cardiac surgery. The aim of this study was to develop, evaluate and validate a new risk score model for GI complications after cardiac surgery. The risk score model, named gastrointestinal complication score (GICS), was developed using prospectively collected data from 5593 patients who underwent 5636 cardiac surgical procedures between 1996 and 2001. The model was validated on 1031 cardiac surgery patients between 2005 and 2006. The scoring system's ability to predict GI complications was estimated by receiver operating characteristic (ROC)-curves and Hosmer–Lemeshow test. Fifty GI complications were identified in 47 patients (0.8%) in the developmental data set and eight (0.8%) in the validation data set. The ROC area in the developmental data set was 0.81 with a good calibration estimated by Hosmer–Lemeshow test (P=0.89). In the validation data set, the area under the curve was 0.83. The estimated probability for the patient to develop a GI complication after cardiac surgery at a GICS ≥15 is >20% and at a GICS ≤5 is <0.4%. Risk stratification according to GICS, specifically developed to predict GI complications after cardiac surgery, showed a good predictive ability.
1. Introduction
Gastrointestinal (GI) complications occur after 0.4–2.9% of cardiac surgery procedures [1–3]. Although infrequent, it is one of the serious complications of cardiac surgery with a high associated morbidity and a mortality rate of 14–63% [4]. It is a challenge to obtain a correct diagnosis because the GI complications frequently present with atypical symptoms, the patients often have multiple underlying diseases and multiple drug therapies, and may be unable to describe symptoms or react to examination due to sedation and analgesia. There is thus a risk of delayed diagnosis and treatment. Various demographic and surgical variables and postoperative events have been suggested as risk factors [1, 2, 5, 6]. In a previous study, our group has identified the following nine risk factors for development of major GI complications: age >80 years, active smoker, need for preoperative inotropic support, New York Heart Association (NYHA) class III–IV, cardiopulmonary bypass >150 min, postoperative atrial fibrillation, postoperative heart failure, reoperation due to bleeding and postoperative vascular complications [7].
Risk stratification plays an important role in cardiac surgical practise worldwide, such as American College of Cardiology/American Heart Association (ACA/AHA) score and Society of Thoracic Surgeons (STS) score [8]. Another risk stratification score, based on a large patient database and widely used is the multinational European System for Cardiac Operative Risk Evaluation (EuroSCORE) [8]. The model was developed for the prediction of operative mortality in the whole context of cardiac surgery, but has also been evaluated in the prediction of direct costs, postoperative complications [9] (including GI complications [10]), and postoperative length of stay [11].
The aim of this study was to develop a risk score model specific for predicting GI complications after cardiac surgery using previously identified risk factors, and to validate the model.
2. Methods
A developmental data set, which served for the construction of the risk model, consisted of data from 6199 adult (≥18 years) patients who underwent 6186 cardiac surgical procedures at the University Hospitals of Lund and Malmö between the years 1996 and 2001. Patients with a missing value in any of the nine risk variables for a GI complication after cardiac surgery (Table 1 ) were excluded. Thus, 5593 patients, undergoing 5636 operations, were included in the analysis. Of the operations, 4000 underwent coronary artery bypass grafting (CABG), 1278 valve or combined valve – CABG procedures, and 358 miscellaneous procedures (including surgery for post-infarction septal rupture, aortic dissection type A and ascending aortic aneurysm). The mean age was 66.6±10.3 years and 4048 patients were men (72%). Data collection, quality checks, and identification of the risk factors associated with GI complications have been described by Andersson et al. [7].
Independent predictors for major gastrointestinal complications after cardiac surgery in the developmental data set and weights (scores) for the risk factors in the GICS model [7]
| Variables | Procedures | Odds ratio | P-value | Score |
| (n=5636) | ||||
| Age >80 years | 292 (5.2) | 2.4 (1.0, 5.8) | 0.050 | 2.5 |
| Active smoker | 491 (8.7) | 2.3 (1.0, 5.5) | 0.049 | 2.5 |
| Inotropic support (preoperative) | 59 (1.1) | 4.0 (1.4, 12) | 0.011 | 4.0 |
| NYHA class III–IV | 2132 (37.8) | 2.0 (1.0, 3.8) | 0.041 | 2.0 |
| Cardiopulmonary bypass time >150 min | 657 (11.7) | 2.5 (1.3, 4.8) | 0.005 | 2.5 |
| Postoperative atrial fibrillation | 1494 (26.5) | 2.4 (1.3, 4.4) | 0.006 | 2.5 |
| Postoperative heart failure | 356 (6.3) | 3.4 (1.6, 7.1) | 0.001 | 3.5 |
| Reoperation due to bleeding | 240 (4.3) | 3.6 (1.6, 7.9) | 0.002 | 3.5 |
| Postoperative vascular complication | 18 (0.3) | 9.3 (1.8, 47) | 0.007 | 9.5 |
| Variables | Procedures | Odds ratio | P-value | Score |
| (n=5636) | ||||
| Age >80 years | 292 (5.2) | 2.4 (1.0, 5.8) | 0.050 | 2.5 |
| Active smoker | 491 (8.7) | 2.3 (1.0, 5.5) | 0.049 | 2.5 |
| Inotropic support (preoperative) | 59 (1.1) | 4.0 (1.4, 12) | 0.011 | 4.0 |
| NYHA class III–IV | 2132 (37.8) | 2.0 (1.0, 3.8) | 0.041 | 2.0 |
| Cardiopulmonary bypass time >150 min | 657 (11.7) | 2.5 (1.3, 4.8) | 0.005 | 2.5 |
| Postoperative atrial fibrillation | 1494 (26.5) | 2.4 (1.3, 4.4) | 0.006 | 2.5 |
| Postoperative heart failure | 356 (6.3) | 3.4 (1.6, 7.1) | 0.001 | 3.5 |
| Reoperation due to bleeding | 240 (4.3) | 3.6 (1.6, 7.9) | 0.002 | 3.5 |
| Postoperative vascular complication | 18 (0.3) | 9.3 (1.8, 47) | 0.007 | 9.5 |
Values in parentheses are percentage for the procedures and 95% confidence intervals for the odds ratio. Statistical analysis by stepwise logistic regression.
GICS, gastrointestinal complication score; NYHA, New York Heart Association.
Independent predictors for major gastrointestinal complications after cardiac surgery in the developmental data set and weights (scores) for the risk factors in the GICS model [7]
| Variables | Procedures | Odds ratio | P-value | Score |
| (n=5636) | ||||
| Age >80 years | 292 (5.2) | 2.4 (1.0, 5.8) | 0.050 | 2.5 |
| Active smoker | 491 (8.7) | 2.3 (1.0, 5.5) | 0.049 | 2.5 |
| Inotropic support (preoperative) | 59 (1.1) | 4.0 (1.4, 12) | 0.011 | 4.0 |
| NYHA class III–IV | 2132 (37.8) | 2.0 (1.0, 3.8) | 0.041 | 2.0 |
| Cardiopulmonary bypass time >150 min | 657 (11.7) | 2.5 (1.3, 4.8) | 0.005 | 2.5 |
| Postoperative atrial fibrillation | 1494 (26.5) | 2.4 (1.3, 4.4) | 0.006 | 2.5 |
| Postoperative heart failure | 356 (6.3) | 3.4 (1.6, 7.1) | 0.001 | 3.5 |
| Reoperation due to bleeding | 240 (4.3) | 3.6 (1.6, 7.9) | 0.002 | 3.5 |
| Postoperative vascular complication | 18 (0.3) | 9.3 (1.8, 47) | 0.007 | 9.5 |
| Variables | Procedures | Odds ratio | P-value | Score |
| (n=5636) | ||||
| Age >80 years | 292 (5.2) | 2.4 (1.0, 5.8) | 0.050 | 2.5 |
| Active smoker | 491 (8.7) | 2.3 (1.0, 5.5) | 0.049 | 2.5 |
| Inotropic support (preoperative) | 59 (1.1) | 4.0 (1.4, 12) | 0.011 | 4.0 |
| NYHA class III–IV | 2132 (37.8) | 2.0 (1.0, 3.8) | 0.041 | 2.0 |
| Cardiopulmonary bypass time >150 min | 657 (11.7) | 2.5 (1.3, 4.8) | 0.005 | 2.5 |
| Postoperative atrial fibrillation | 1494 (26.5) | 2.4 (1.3, 4.4) | 0.006 | 2.5 |
| Postoperative heart failure | 356 (6.3) | 3.4 (1.6, 7.1) | 0.001 | 3.5 |
| Reoperation due to bleeding | 240 (4.3) | 3.6 (1.6, 7.9) | 0.002 | 3.5 |
| Postoperative vascular complication | 18 (0.3) | 9.3 (1.8, 47) | 0.007 | 9.5 |
Values in parentheses are percentage for the procedures and 95% confidence intervals for the odds ratio. Statistical analysis by stepwise logistic regression.
GICS, gastrointestinal complication score; NYHA, New York Heart Association.
2.1. Patients and study design
A validation data set, including 1123 consecutive adult patients undergoing 1128 cardiac surgery procedures at the University Hospital of Lund between July 2005 and June 2006, was prospectively collected. Patients with a missing value (n=75) in the outcome variable GI complication were excluded, as were patients who had cardiac transplantation and required immunotherapy (n=11) or coronary bypass grafting without extracorporeal circulation (n=11). Thus, 1027 patients, undergoing 1031 operations, were included in the analysis. Of the operations, 620 underwent CABG, 288 valve or combined valve – CABG procedures, and 123 miscellaneous procedures (including surgery for post-infarction septal rupture, aortic dissection type A and ascending aortic aneurysm). The mean age was 67.1±11.1 and most patients were men (73%). The patient record form contained pre-, per- and postoperative variables including the 18 pre- and peroperative risk factors defined according to EuroSCORE and the nine risk factors for development of GI complications, Table 1. Congestive heart failure was defined as the presence of any three of the following: dyspnoea, rales from pulmonary congestion, peripheral oedema, cardiomegaly or interstitial oedema on chest radiography. Postoperative heart failure was diagnosed if inotropic support was required for >48 h and/or use of intra-aortic balloon pump after surgery. Postoperative neurological dysfunction was diagnosed in the event of permanent or transient stroke or postoperative confusion. Postoperative renal failure was defined as a serum creatinine level above 200 μmol/l and/or need for dialysis.
In 0.6% of the total data points, missing values were replaced using the probability imputation technique [12] before the risk score was calculated. There was accurate documentation of data including the outcome, i.e. GI complication, in all included cases and no patient was lost to follow-up. GI complications were defined according to a modification of the definitions made by Christenson et al. [2], Table 2 .
Definitions of major gastrointestinal complications, modified from Christensen et al. [2]
| Definitions | |
| Major GI complications | |
| Upper GI bleeding | Haematemesis and/or melaena and transfusion |
| of ≥2 units of blood | |
| Lower GI bleeding | Rectal bleeding with transfusion of ≥2 units |
| of blood | |
| Perforation | Diagnosed by X-ray or at laparotomy or autopsy |
| Intestinal ischaemia | Found at laparotomy/laparoscopy or endoscopy |
| or autopsy | |
| Liver failure | Without any other major abdominal complications, |
| manifested by laboratory data | |
| [aspartateaminotransferase (ASAT) and | |
| alanineaminotransferase (ALAT) >50 times | |
| upper normal level] | |
| Pancreatitis | Clinical features and amylase >3 times upper |
| normal level and/or typical radiological findings | |
| Acute cholecystitis | Found at ultrasonography and/or laparotomy |
| Paralytic ileus | Abdominal distension and typical X-ray findings |
| Definitions | |
| Major GI complications | |
| Upper GI bleeding | Haematemesis and/or melaena and transfusion |
| of ≥2 units of blood | |
| Lower GI bleeding | Rectal bleeding with transfusion of ≥2 units |
| of blood | |
| Perforation | Diagnosed by X-ray or at laparotomy or autopsy |
| Intestinal ischaemia | Found at laparotomy/laparoscopy or endoscopy |
| or autopsy | |
| Liver failure | Without any other major abdominal complications, |
| manifested by laboratory data | |
| [aspartateaminotransferase (ASAT) and | |
| alanineaminotransferase (ALAT) >50 times | |
| upper normal level] | |
| Pancreatitis | Clinical features and amylase >3 times upper |
| normal level and/or typical radiological findings | |
| Acute cholecystitis | Found at ultrasonography and/or laparotomy |
| Paralytic ileus | Abdominal distension and typical X-ray findings |
GI, gastrointestinal.
Definitions of major gastrointestinal complications, modified from Christensen et al. [2]
| Definitions | |
| Major GI complications | |
| Upper GI bleeding | Haematemesis and/or melaena and transfusion |
| of ≥2 units of blood | |
| Lower GI bleeding | Rectal bleeding with transfusion of ≥2 units |
| of blood | |
| Perforation | Diagnosed by X-ray or at laparotomy or autopsy |
| Intestinal ischaemia | Found at laparotomy/laparoscopy or endoscopy |
| or autopsy | |
| Liver failure | Without any other major abdominal complications, |
| manifested by laboratory data | |
| [aspartateaminotransferase (ASAT) and | |
| alanineaminotransferase (ALAT) >50 times | |
| upper normal level] | |
| Pancreatitis | Clinical features and amylase >3 times upper |
| normal level and/or typical radiological findings | |
| Acute cholecystitis | Found at ultrasonography and/or laparotomy |
| Paralytic ileus | Abdominal distension and typical X-ray findings |
| Definitions | |
| Major GI complications | |
| Upper GI bleeding | Haematemesis and/or melaena and transfusion |
| of ≥2 units of blood | |
| Lower GI bleeding | Rectal bleeding with transfusion of ≥2 units |
| of blood | |
| Perforation | Diagnosed by X-ray or at laparotomy or autopsy |
| Intestinal ischaemia | Found at laparotomy/laparoscopy or endoscopy |
| or autopsy | |
| Liver failure | Without any other major abdominal complications, |
| manifested by laboratory data | |
| [aspartateaminotransferase (ASAT) and | |
| alanineaminotransferase (ALAT) >50 times | |
| upper normal level] | |
| Pancreatitis | Clinical features and amylase >3 times upper |
| normal level and/or typical radiological findings | |
| Acute cholecystitis | Found at ultrasonography and/or laparotomy |
| Paralytic ileus | Abdominal distension and typical X-ray findings |
GI, gastrointestinal.
2.2. Statistical analysis
The odds ratios for the nine risk factors in the developmental data set were used to construct the score for each variable in the final model, Table 1. The calibration of the model was assessed by comparing the observed and expected outcome for equally sized quantiles of risk, by using Hosmer–Lemeshow goodness-of-fit test. The discriminatory power of the two models was evaluated by calculation of the area under the receiver operating characteristic (ROC)-curves, with 95% confidence limits. To compare the areas under the resulting ROC-curves the non-parametric approach described by DeLong et al. was used [13]. Statistical analyses and graphs were performed with Stata MP version 10.1, 2009 statistical package for Mac OS X (Stata Corporation, College Station, Texas, USA).
3. Results
3.1. Patient population
Fifty GI complications were identified in 47 patients (incidence 0.8%) in the developmental data set. Thirteen of these 47 patients died within 30 days (28%). The most common complication was upper GI bleeding (16 patients), and intestinal ischaemia was the most lethal one (8/10). In the validation data set (n=1031), eight major GI complications in eight patients were identified (incidence 0.8%). The most common complication was upper GI bleeding (five patients). One patient died within 30 days after cardiac surgery.
3.2. Performance and predictive accuracy for the model
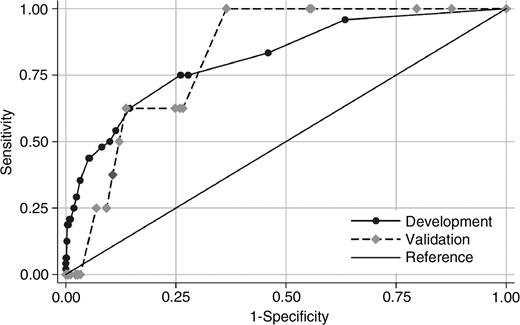
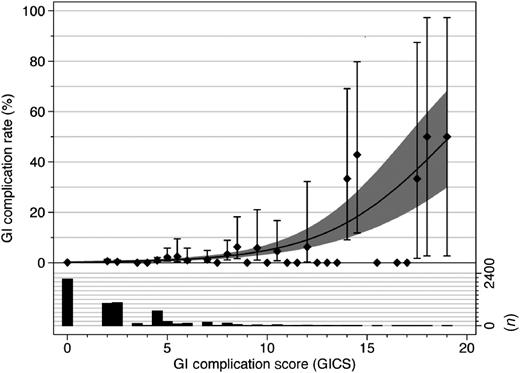
The weight attributed to each variable in the new risk scoring model [gastrointestinal complication score (GICS)], specific for GI complications after cardiac surgery was obtained from the odds ratio for each risk variable identified in the developmental data set, Table 1. The Hosmer–Lemeshow goodness-of-fit test was used to test the calibration of the model. The predicted accuracy was calculated after dividing the patients into five different risk groups. The P-values of 0.89 in the developmental set, Table 3 and 0.28 in the validation set indicated a good calibration of the model. The area under the ROC-curve (Fig. 1 ) was 0.81 [95% confidence interval (CI) 0.75–0.88] in the developmental set and 0.83 (95% CI 0.74–0.91) in the validation set (P=0.839). At a GICS of 5 the sensitivity was 56% and specificity 88%, and at a score of 15 it was 13% and 99.8%, respectively. The probability of a GI complication after cardiac surgery is >20% at a GICS of 15 or above and <0.4% at a GICS of 5 or below, Fig. 2 .
GICS risk stratification model: predicted vs. observed gastrointestinal (GI) complications after cardiac surgery in the developmental data set
| Quantiles of risk | Number of | Predicted GI | Observed GI | ||
| operations | complications | complications | |||
| n | % | n | % | ||
| First | 1310 | 4 | 0.31 | 5 | 0.38 |
| Second | 1290 | 6 | 0.47 | 4 | 0.31 |
| Third | 1176 | 8 | 0.67 | 7 | 0.60 |
| Fourth | 919 | 9 | 0.96 | 11 | 1.20 |
| Fifth | 941 | 20 | 2.14 | 20 | 2.13 |
| Total | 5636 | 47 | 0.83 | 47 | 0.83 |
| Hosmer–Lemeshow | 0.61 | ||||
| χ2 (3 df) | |||||
| P>χ2 | 0.89 | ||||
| Quantiles of risk | Number of | Predicted GI | Observed GI | ||
| operations | complications | complications | |||
| n | % | n | % | ||
| First | 1310 | 4 | 0.31 | 5 | 0.38 |
| Second | 1290 | 6 | 0.47 | 4 | 0.31 |
| Third | 1176 | 8 | 0.67 | 7 | 0.60 |
| Fourth | 919 | 9 | 0.96 | 11 | 1.20 |
| Fifth | 941 | 20 | 2.14 | 20 | 2.13 |
| Total | 5636 | 47 | 0.83 | 47 | 0.83 |
| Hosmer–Lemeshow | 0.61 | ||||
| χ2 (3 df) | |||||
| P>χ2 | 0.89 | ||||
GICS, gastrointestinal complication score.
GICS risk stratification model: predicted vs. observed gastrointestinal (GI) complications after cardiac surgery in the developmental data set
| Quantiles of risk | Number of | Predicted GI | Observed GI | ||
| operations | complications | complications | |||
| n | % | n | % | ||
| First | 1310 | 4 | 0.31 | 5 | 0.38 |
| Second | 1290 | 6 | 0.47 | 4 | 0.31 |
| Third | 1176 | 8 | 0.67 | 7 | 0.60 |
| Fourth | 919 | 9 | 0.96 | 11 | 1.20 |
| Fifth | 941 | 20 | 2.14 | 20 | 2.13 |
| Total | 5636 | 47 | 0.83 | 47 | 0.83 |
| Hosmer–Lemeshow | 0.61 | ||||
| χ2 (3 df) | |||||
| P>χ2 | 0.89 | ||||
| Quantiles of risk | Number of | Predicted GI | Observed GI | ||
| operations | complications | complications | |||
| n | % | n | % | ||
| First | 1310 | 4 | 0.31 | 5 | 0.38 |
| Second | 1290 | 6 | 0.47 | 4 | 0.31 |
| Third | 1176 | 8 | 0.67 | 7 | 0.60 |
| Fourth | 919 | 9 | 0.96 | 11 | 1.20 |
| Fifth | 941 | 20 | 2.14 | 20 | 2.13 |
| Total | 5636 | 47 | 0.83 | 47 | 0.83 |
| Hosmer–Lemeshow | 0.61 | ||||
| χ2 (3 df) | |||||
| P>χ2 | 0.89 | ||||
GICS, gastrointestinal complication score.
The ROC-curves from the developmental dataset (solid line) and the validation dataset (dashed line) for the GICS model. There is no significant difference between the areas under the curves, χ2=0.04 (P=0.839). ROC, receiver operating characteristic; GICS, gastrointestinal complication score.
Percentage of patients with a GI complication after cardiac surgery (left y-axis) for each GICS risk group (x-axis). Predicted GI complication (solid line) with 95% confidence intervals (shadowed area); observed percentage of GI complications (diamond) with 95% confidence intervals (vertical bars). The histogram shows the number of patients (right y-axis) in each risk group. GI, gastrointestinal.
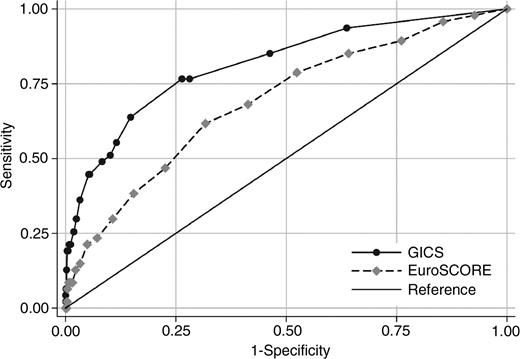
The Hosmer–Lemeshow goodness-of-fit gave a P-value of 0.66 for the EuroSCORE to predict a GI complication after cardiac surgery in the developmental set. The area under the ROC-curve was 0.71 (95% CI 0.63–0.79), which was significantly smaller compared with GICS (P<0.0006), Fig. 3 . In the validation set, the area under the ROC-curve was larger for GICS than for the EuroSCORE (0.74; 95% CI 0.53–0.94), but the difference did not reach statistical significance (P=0.432) in this smaller dataset. The Hosmer–Lemeshow goodness-of-fit gave a P-value of 0.75 for the EuroSCORE.
The ROC-curves from the developmental dataset. The GICS (solid line) and the EuroSCORE (dashed line), risk stratification models. The area under the curve for the GICS model is larger compared with the EuroSCORE model, χ2=11.9 (P<0.0006). GICS, gastrointestinal complication score; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ROC, receiver operating characteristic.
To evaluate the risk models specificity, the model was tested on four other outcomes. The ROC area for postoperative myocardial infarction was 0.72 (95% CI 0.68–0.76), neurological dysfunction 0.70 (95% CI 0.66–0.72), infection 0.64 (95% CI 0.61–0.67) and renal failure 0.74 (95% CI 0.71–0.77).
4. Discussion
This study aimed to develop a risk score model specific for prediction of GI complications after cardiac surgery and to validate the model. Based on previously identified independent risk factors for GI complications after cardiac surgery [7] the model, GICS, was developed. GICS showed a good ability to predict these complications, both in the developmental data set and in a validation set, and predicted GI complications better than other postoperative complications.
Identification of postoperative GI complications in cardiac surgery patients is extremely important but can be difficult and demanding. Several authors have stressed the importance of early recognition of GI complications [1, 7] with a low threshold to proceed to exploratory laparotomy, in order to minimize delay of ‘source control’, thereby potentially diminishing intraperitoneal sepsis and multisystem organ failure. However, patient evaluation is difficult. Patients are often mechanically ventilated and sedated, thereby masking usual symptoms and signs, reinforcing the message that the clinician must suspect a GI complication in any patient with abdominal pain or tenderness. Although the cardiac status needs to be taken into consideration, this will in many patients be better than before the heart surgery, making them able to withstand general anaesthesia required for laparotomy.
A diagnostic tool, which can be used bedside repeatedly postoperatively, as help in surgical decision-making was the desired product from the present work. It can be used routinely in postoperative patients or in the clinical situation when suspicion of the complication arises, but it is not developed to be used in preoperative risk stratification.
Several of the risk factors in the present scoring model are related to the underlying problem in most GI complications, namely low splanchnic blood flow. Pre-existing atherosclerosis and atheroembolism, the latter perhaps an underestimated factor [14], are also connected to the present scoring model.
No specific risk score model for the dreaded and diagnostically challenging complications in the GI tract after cardiac surgery does hitherto exist. The widely used multinational EuroSCORE, based on a large patient database and specifically developed for the prediction of operative mortality has, however, also been evaluated in the prediction of other clinically important outcomes, including postoperative complications [9]. In three previous publications EuroSCORE was unable to predict GI complications, both in a selected group with CABG [15] and in a group undergoing various cardiothoracic operations. In a fourth study, however, EuroSCORE was able to predict GI complications after heart valve surgery [10]. The overall poor ability for EuroSCORE to predict GI complications was confirmed in the present study, when compared with the new risk model specifically constructed for predicting GI complications. In the developmental data set, the difference reached statistical significance.
A limitation in the present study, although it is validated on a separate data set than it was constructed on, is that the study refers to a single-centre regional database. It would be of importance to further evaluate the system, and to prove its generalizability across diverse institutions and countries. A statistically significant difference between GICS and EuroSCORE in the validation material could not be proved. This could be explained by the proportionately smaller validation material, with a low total incidence of the complication studied. The predicted power of the model (ROC area 0.8) in the present study is comparable to currently clinically used mortality prediction models [8]. Another aspect to be aware of is that since the incidence of GI complications are quite low, most studies, including the present one, do not analyze these in different subgroups, and therefore not fully taking into account the possibly different pathophysiological backgrounds.
In conclusion, the present study presents a new and, to our knowledge, the first risk score model specifically developed, to predict GI complications after cardiac surgery. GICS is constructed to be easy to use bedside. In order to reduce morbidity and mortality for the patients contracting these complications it is important to set the correct diagnosis and to provide proper treatment. A greater awareness of risk factors that contribute to GI complications can lead to more careful patient management. These patients would benefit from close surveillance for early diagnosis, which also should lead to an aggressive treatment approach. The model helps to create a better framework for surgical decision-making for this patient group.