Abstract
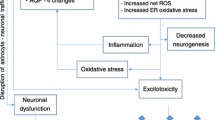
Thiamine deficiency results in Wernicke’s encephalopathy and is commonly encountered in chronic alcoholism, gastrointestinal diseases, and HIV AIDS. The earliest metabolic consequence of thiamine deficiency is a selective loss in activity of the thiamine diphosphate-dependent enzyme α-ketoglutarate dehydrogenase (α-KGDH), a rate-limiting tricarboxylic acid cycle enzyme. Thiamine deficiency is characterized neuropathologically by selective neuronal cell death in the thalamus, pons, and cerebellum. The cause of this region-selective neuronal loss is unknown, but mechanisms involving cellular energy failure, focal lactic acidosis, and NMDA receptor-mediated excitotoxicity have classically been implicated. More recently, evidence supports a role for oxidative stress. Evidence includes increased endothelial nitric oxide synthase, nitrotyrosine deposition, microglial activation, and lipid peroxidation. Reactive oxygen species production results in decreased expression of astrocytic glutamate transporters and decreased activities of α-KGDH, resulting in an amplification of cell death mechanisms in thiamine deficiency.
Similar content being viewed by others
References
Harper C.G. and Butterworth R.F. (1997) Nutritional and metabolic disorders, in Greenfield’s Neuropathology, Graham D.I. and Lantos P.L. eds., Arnold, London, pp. 601–655.
Peters R.A. (1936) The biochemical lesion in vitamin B1 deficiency. Application of modern biochemical analysis in its diagnosis. Lancet 1, 1161–1164.
Hécoux M. and Butterworth R.F. (1995) Regional alterations of thiamine phosphate esters and of thiamine diphosphate-dependent enzymes in relation to function in experimental Wernicke’s encephalopathy. Neurochem. Res. 20, 87–93.
Butterworth R.F. and Hécoux M. (1989) Effect of pyrithiamine treatment and subsequent thiamine rehabilitation on regional cerebral amino acids and thiamine-dependent enzymes. J. Neurochem. 52, 1079–1084.
Butterworth R.F., Kril J.J., and Harper C. (1993) Thiamine-dependent enzyme changes in brain of alcoholics: relationship to Wernicke-Korsakoff Syndrome. Alcohol Clin. Exp. Res. 17, 1084–1088.
Parker W.D., Jr., Haas R., Stumpf D.A., Parks J., Eguren L.A., and Jackson C. (1984) Brain mitochondrial metabolism in experimental thiamine deficiency. Neurology 34, 1477–1481.
Pannunzio P., Hazell A.S., Pannunzio M., Rama Rao K.V., and Butterworth R.F. (2000) Thiamine deficiency results in metabolic acidosis and energy failure in cerebellar granule cells: an in vitro model for the study of cell death mechanisms in Wernicke’s encephalopathy. J. Neurosci. Res. 62, 286–292.
Bettendorff L., Sluse F., Goessens G., Wins P., and Grisar T. (1995) Thiamine deficiency-induced partial necrosis and mitochondrial uncoupling in neuroblastoma cells are rapidly reversed by addition of thiamine. J. Neurochem. 65, 2178–2184.
Zhang S.X., Weilersbacher G.S., Henderson S.W., Corso T., Olney J.W., and Langlais P.J. (1995) Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J. Neuropathol. Exp. Neurol. 54, 255–267.
Aikawa H., Watanabe I.S., Furuse T., et al. (1984) Low energy level in thiamine-deficient encephalopathy. J. Neuropathol. Exp. Neurol. 43, 276–287.
Hakim A.M. (1984) The induction and reversibility of cerebral acidosis in thiamine deficiency. Ann. Neurol. 16, 673–679.
Hazell A.S., Butterworth R.F., and Hakim A.M. (1993) Cerebral vulnerability is associated with selective increase in extracellular glutamate concentration in experimental thiamine deficiency. J. Neurochem. 61, 1155–1158.
Hazell A.S., Rao K.V., Danbolt N.C., Pow D.V., and Butterworth R.F. (2001) Selective downregulation of the astrocyte glutamate transporters GLT-1 and GLAST within the medial thalamus in experimental Wernicke’s encephalopathy. J. Neurochem. 78, 560–568.
Langlais P.J. and Mair R.G. (1990) Protective effects of the glutamate antagonist MK-801 on pyrithiamine-induced lesions and amino acid changes in rat brain. J. Neurosci. 10, 1664–1674.
Todd K.G. and Butterworth R.F. (1998) Evaluation of the role of NMDA-mediated excitotoxicity in the selective neuronal loss in experimental Wernicke encephalopathy. Exp. Neurol. 149, 130–138.
Langlais P.J., Anderson G., Guo S.X., and Bondy S.C. (1997) Increased cerebral free radical production during thiamine deficiency. Metab. Brain Dis. 12, 137–143.
Cullen K.M. and Halliday G.M. (1995) Mechanisms of cell death in cholinergic basal forebrain neurons in chronic alcoholics. Metab. Brain Dis. 10, 81–91.
Gibson G.E. and Zhang H. (2002) Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem. Int. 40, 493–504.
Kruse M., Navarro D., Desjardins P., and Butterworth R.F. (2004) Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem. Int. 45, 49–56.
Todd K.G. and Butterworth R.F. (1999) Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia 25, 190–198.
Collins G.H. (1967) Glial cell changes in the brain stem of thiamine-deficient rats. Am. J. Pathol. 50, 791–814.
Robertson D.M., Wasan S.W., and Skinner D.B. (1968) Ultrastructure features of early brain stem lesions of thiamine-deficient rats. Am. J. Pathol. 52, 1081–1087.
Tellez I. and Terry R.D. (1968) Fine structure of the early changes in the vestibular nuclei of the thiamine-deficient rat. Am. J. Pathol. 52, 777–794.
Victor M., Adams R.D., and Collins G.H. (1989) The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition, 2nd ed., F.A. Davies Philadelphia, pp. 61–110.
Gehrmann J., Bonnehoh P., Miyazawa T., Hossmann K.A., and Kreutzberg G.W. (1992) Immunocytochemical study of an early microglial activation in ischemia. J. Cereb. Blood Flow Metab. 12, 257–269.
Okeda R., Taki K., Ikari R., and Funata N. (1995) Vascular changes in acute Wernicke’s encephalopathy. Acta Neuropathol. (Berl.) 89, 420–424.
Calingasan N.Y., Huang P.L., Chun H.S., Fabian A., and Gibson G.E. (2000) Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J. Neuropathol. Exp. Neurol. 59, 207–217.
Bolanos J.P. and Almeida A. (1999) Roles of nitric oxide in brain hypoxia-ischemia. Biochem. Biophys. Acta 1411, 415–436.
Estevez A.G., Spear N., Thompson J.A., et al. (1998) Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J. Neurosci. 18, 3708–3714.
Matsushima K., MacManus J.P., and Hakim A.M. (1997) Apoptosis is restricted to the thalamus in thiamine-deficient rats. Neuroreport 8, 867–870.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desjardins, P., Butterworth, R.F. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol 31, 17–25 (2005). https://doi.org/10.1385/MN:31:1-3:017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:31:1-3:017