Abstract
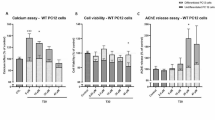
The ability of nicotine to induce a cytoprotective or neuroprotective action occurs through several down-stream mechanisms. One possibility is that the drug increases the expression of tyrosine kinase A (TrkA) nerve growth factor (NGF) receptors. Certain β-amyloid peptides (e.g., Aβ1–42) have been shown to bind with high affinity to α7 nicotinic receptors and thus interfere with a potentially neurotrophic influence. Treatment of differentiated PC-12 cells with nicotine produced a concentration-dependent increase in cell-surface TrkA receptors that occurred concomitantly with cytoprotection. The effect of nicotine was blocked by either of the α7 receptor antagonists α-bungarotoxin (α-BTX) or methyllycaconatine. The cytoprotective action of nicotine also was inhibited by pretreatment with 10–100 nM Aβ1–42. Nicotine also was administered (four injections of 30 µg, spaced evenly over 24 h) to rats by direct injection into a lateral cerebral ventricle. Brain TrkA expression was increased significantly in hippocampus and entorhinal cortex (up to 32% above control), with no changes found in cerebral cortex or hypothalamus. The nicotine-induced increases in TrKA expression in hippocampus and entorhinal cortex were significantly inhibited by 10 µg α-BTX or by 10 nmol Aβ1–42. Therefore, physiologically relevant concentrations of Aβ1–42 can prevent nicotine-induced TrkA receptor expression in brain regions containing cholinergic neurons susceptible to the neurotoxicity associated with Alzheimer’s disease.
Similar content being viewed by others
References
Barbacid M. (1994) The Trk family of neurotrophin receptors. J. Neurobiol. 25, 1386–1403.
Blumenthal E. M., Conroy W. G., Romano S. J., Kassner P. D., and Berg D. K. (1997) Detection of functional nicotinic receptors blocked by α-bungarotoxin on PC-12 cells and dependence of their expression on post-translational events. J. Neurosci. 17, 6094–6104.
Boissiere F., Hunot S., Faucheux B., Hersh L. B., Agid Y., and Hirsch E. C. (1997) Trk neurotrophin receptors in cholinergic neurons of patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 8, 1–8.
Buccafusco J. J. (2004) Neuronal nicotinic receptor subtypes: defining therapeutic targets. Mol. Interventions 4, 285–295.
Capsoni S., Ugolini G., Comparini A., Ruberti F., Berardi N., and Cattaneo A. (2000) Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 97, 6826–6831.
Carlson N. G., Bacchi A., Rogers S. W., et al. (1998) Nicotine blocks TNF-alpha-mediated neuroprotection to NMDA by an alpha-bungarotoxin-sensitive pathway. J. Neurobiol. 35, 29–36.
Chao M. V. (1992) Neurotrophin receptors: a window into neuronal differentiation. Neuron 9, 583–593.
Clary D. O., Weskamp G., Austin L. R., and Reichardt L. F. (1994) TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol. Biol. Cell 5, 549–563.
Craft J. M., Van Eldik L. J., Zasadzki M., Hu W., and Watterson D.M. (2004a) Aminopyridazines attenuate hippocampus-dependent behavioral deficits induced by human β-amyloid in a murine model of neuroin-flammation. J. Mol. Neurosci. 24, 115–122.
Craft J. M., Watterson D. M., Frautschy S. A., and Van Eldik L. J. (2004b) Aminopyridazines inhibit β-amyloid-induced glial activation and neuronal damage in vivo. Neurobiol. Aging 25, 1283–1292.
Dajas-Bailador F. A., Lima P. A., and Wonnacott S. (2000) The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology 39, 2799–2807.
Donnelly-Roberts D. L., Xue I. C., Arneric S. P., and Sullivan J. P. (1996) In vitro neuroprotective properties of the novel cholinergic channel activator (ChCA), ABT-418. Brain Res. 719, 36–44.
Drisdel R. C. and Green W. N. (2000) Neuronal α-bungarotoxin receptors are α7 subunit homomers. J. Neurosci. 20, 133–139.
Etienne P., Robitaille Y., Wood P., Gauthier S., Nair N. P., and Quirion R. (1986) Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer’s disease. Neuroscience 19, 1279–1291.
Frautschy S. A., Fusheng Y., Calderón L., and Cole G. M. (1996) Rodent Models of Alzheimer’s disease: rat Aβ infusion approaches to amyloid deposits. Neurobiol. Aging, 17, 311–321.
Gattu M., Pauly J. R., Boss K., Summers J. B., and Buccafusco J. J. (1997) Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors. I. Brain Res. 771, 89–103.
Gearhart D. A., Middlemore M. L., and Terry A. V. (2004) Development of ELISA methods to quantify cholinergic markers in rat brain lysates. Soc. Neurosci. Abstr. 30, Program no. 695.8.
Harkany T., Hortobagyi T., Sasva’ri M., et al. (1999) Neuroprotective approaches in experimental models of β-amyloid neurotoxicity: relevance to Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 23, 963–1008.
Hartikka J. and Hefti F. (1988) Comparison of nerve growth factor’s effects on development of septum, striatum, and nucleus basalis cholinergic neurons in vitro. J Neurosci. Res. 21, 352–364.
Hefti F. and Knusel B. (1988) Chronic administration of nerve growth factor and other neurotrophic factors to the brain. Neurobiol. Aging 9, 689–690.
Hellstrom-Lindahl E., Court J., Keverne J., et al. (2004a) Nicotine reduces A beta in the brain and cerebral vessels of APPsw mice. Eur. J. Neurosci. 19, 2703–2710.
Hellstrom-Lindahl E., Mousavi M., Ravid R., and Nordberg A. (2004b) Reduced levels of Abeta 40 and Abeta 42 in brains of smoking controls and Alzheimer’s patients. Neurobiol. Dis. 15, 351–360.
Henderson L. P., Gdovin M. J., Liu C., Gardner P. D., and Maue R. A. (1994) Nerve growth factor increases nicotinic ACh receptor gene expression and current density in wild-type and protein kinase A-deficient PC-12 cells. J. Neurosci. 14, 1153–1163.
Ikarashi Y., Harigaya Y., Tomidokoro Y., et al. (2004) Decreased level of brain acetylcholine and memory disturbance in APPsw mice. Neurobiol. Aging 25, 483–490.
Iverson L. L., Mortishire-Smith R. J., Pollack S. J., and Shearman M. S. (1995) The toxicity in vitro of β-amyloid protein. Biochem. J. 311, 1–16.
Jonnala R. R. and Buccafusco J. J. (2001) Relationship between cell surface α7 nicotinic receptor expression and neuroprotection induced by several nicotinic receptor agonists. J. Neurosci. Res. 66, 565–572.
Jonnala R. R., Terry A. V. Jr., and Buccafusco J. J. (2002) Nicotine increases the expression of high affinity nerve growth factor receptors both in vitro and in vivo. Life Sci. 70, 1543–1554.
Jonnala R. R., Graham J. H. III, Terry A. V. Jr., Beach J. W., Young J. A., and Buccafusco J. J. (2003) Relative levels of cytoprotection produced by analogs of choline and the role of α7-nicotinic acetylcholine receptors. Synapse 47, 262–269.
Kaneko S., Maeda T., Kume T., et al. (1997) Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors. Brain Res. 765, 135–140.
Kihara T., Shimohama S., Urushitani M., et al. (1998) Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain Res. 792, 331–334.
Kim H. J., Chae S. C., Lee D. K., et al. (2003) Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 17, 118–120.
Kim S. H., Kim Y. K., Jeong S. J., Haass C., Kim Y. H., and Suh Y. H. (1997) Enhanced release of secreted form of Alzheimer’s amyloid precursor protein from PC-12 cells by nicotine. Mol. Pharmacol. 52, 430–436.
Knusel B. and Hefti F. (1988) Development of cholinergic pedunculopontine neurons in vitro: comparison with cholinergic septal cells and response to nerve growth factor, ciliary neuronotrophic factor, and retinoic acid. J Neurosci. Res. 21, 365–375.
Kromer L. F. (1987) Nerve growth factor treatment after brain injury prevents neuronal death. Science 235, 214–216.
Lehericy S., Hirsch E. C., Cervera-Pierot P., et al. (1993) Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J. Comp. Neurol. 330, 15–31.
Li X. D. and Buccafusco J. J. (2003) Effect of beta-amyloid peptide 1–42 on the cytoprotective action mediated by alpha7 nicotinic acetylcholine receptors in growth factor-deprived differentiated PC-12 cells. J. Pharmacol. Exp. Ther. 307, 670–675.
Li X. D. and Buccafusco J. J. (2004) Role of alpha7 nicotinic acetylcholine receptors in the pressor response to intracerebroventricular injection of choline: blockade by amyloid peptide Abeta1–42. J. Pharmacol. Exp. Ther. 309, 1206–1212.
Martin E. J., Panicker K. S., King M. A., Deyrup M., Hunter B. E., and Meyer E.M. (1994) Cytoprotective of 2, 4-dimethoxybenzylidene anabaseine in differentiated PC-12 cells and septal cholinergic neurons. Drug Dev. Res. 31, 127–134.
Mobley W. C., Rutkowski J. L., Tennekoon G. I., Gemski J., Buchanan K., and Johnston M. V. (1986) Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 387, 53–62.
Morimoto K. and Oda T. (2003) Kainate exacerbates beta-amyloid toxicity in rat hippocampus. Neurosci. Lett. 340, 242–244.
Mufson E. J., Lavine N., Jaffar S., Kordower J. H., Quirion R., and Saragovi H. U. (1997) Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer’s disease. Exp. Neurol. 146, 91–103.
Nakamura T., Shoji M., Harigaya Y., et al. (1994) Amyloid beta protein levels in cerebrospinal fluid are elevated in early-onset Alzheimer’s disease. Ann. Neurol. 36, 903–911.
Nordberg A. (2001) Nicotinic receptor abnormalities of Alzheimer’s disease: therapeutic implications. Biolog. Psychiatry 49, 200–210.
O’Neill M. J., Murray T. K., Lakics V., Visanji N. P., and Duty S. (2002) The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr. Drug Targets CNS Neurol. Disord. 1, 399–411.
Paterson D. and Nordberg A. (2000) Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 61, 75–111.
Perry E. K., Martin-Ruiz C. M., and Court J. A. (2001) Nicotinic receptor subtypes in human brain related to aging and dementia. Alcohol 24, 63–68.
Pitchford S., De Moor K., and Glaeser B. S. (1995) Nerve growth factor stimulates rapid metabolic responses in PC-12 cells. Am. J. Physiol. 268, C939-C943.
Rangwala F., Drisdel R. C., Rakhilin S., et al. (1997) Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J. Neurosci. 17, 8201–8212.
Seguela P., Wadiche J., Dineley-Miller K., Dani J. A., and Patrick J. W. (1993) Molecular cloning, functional properties and distribution of rat brain α7: a nicotinic cation highly permeable to calcium. J. Neurosci. 13, 595–604.
Shaw S., Bencherif M., and Marrero M. B (2002) Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-1-42 amyloid. J. Biol. Chem. 277, 44,920–44,924.
Shimohama S. and Kihara T. (2001) Nicotinic receptor-mediated protection against beta-amyloid neurotoxicity. Biol. Psychiatry 49, 233–239.
Snider W. D. (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77, 627–638.
Wang, H. Y., Lee D. H. S., D’Andrea M. R., Peterson P. A., and Shank R. P. (1999) β-amyloid1–42 binds to α7 nicotinic acetylcholine receptor with high affinity. J. Biol. Chem. 275, 5626–5632.
Wang H. Y., Lee D. H. S., Davis C. B., and Shank R. P. (2000) Amyloid peptide Aβ1–42 binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. J. Neurochem. 75, 1155–1161.
Whitehouse P. J., Price D. L., Clark A. W., Coyle J. T., and DeLong M. R. (1981) Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126.
Yang X.-H. and Buccafusco J. J. (1994) Effect of chronic central treatment with the acetylcholine analog methylcarbamylcholine on cortical nicotinic receptors: correlation between receptor changes and behavioral function. J. Pharmacol. Exp. Ther. 271, 651–659.
Zamani M. R., Allen Y. S., Owen G. P., and Gray J. A. (1997) Nicotine modulates the neurotoxic effect of β-amyloid protein (25–35) in hippocampal cultures. Neuroreport 8, 513–517.
Zanardi A., Leo G., Biagini G., and Zoli M. (2002) Nicotine and neurodegeneration in ageing. Toxicol. Lett. 127, 207–215.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X.D., Arias, E., Jonnala, R.R. et al. Effect of amyloid peptides on the increase in TrkA receptor expression induced by nicotine in vitro and in vivo. J Mol Neurosci 27, 325–336 (2005). https://doi.org/10.1385/JMN:27:3:325
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:27:3:325