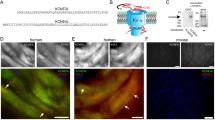
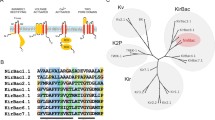
Abstract
Accessory subunits are an essential feature of voltage-gated potassium (Kv) channels. They determine trafficking to the plasma membrane, surface expression, gating, permeation, and pharmacology. At least three distinct classes of accessory subunits including the KCNE family can regulate Kv channel function. KCNE genes encode integral membrane proteins with a single transmembrane domain. KCNE genes span the eukaryotic kingdom and, in mutated form, can cause acquired and congenital disease. Here we review genetic, physiological, and biophysical aspects of KCNE proteins with particular emphasis on the Caenorhabditis elegans subfamily.
Similar content being viewed by others
References
Takumi, T., Ohkubo, H., and Nakanishi, S. (1988) Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science 242, 1042–1045.
Hausdorff, S. F., Goldstein, S. A., Rushin, E. E., and Miller, C. (1991) Functional characterization of a minimal K+ channel expressed from a synthetic gene. Biochemistry 30, 3341–3346.
Abbott, G., Sesti, F., Splawski, I., et al. (1999) MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97, 175–187.
Anantharam, A., Lewis, A., Panaghie, G., et al. (2003) RNA interference reveals that endogenous Xenopus MinK-related peptides govern mammalian K+channel function in oocyte expression studies. J. Biol. Chem. 278, 11,739–11,745.
Bianchi, L., Kwok, S. M., Driscoll, M., and Sesti, F., (2003) A potassium channel-MiRP complex controls neurosensory function in Caenorhabditis elegans. J. Biol. Chem. 278, 12415–12424.
Park, K. H., Hernandez, L., Cai, S. Q., Wang, Y., and Sesti, F. (2005) A family of K+ channel ancillary subunits regulate taste sensitivity in Caenorhabditis elegans. J. Biol. Chem. 280, 21,893–21,899.
Piccini, M., Vitelli, F., Seri, M. et al. (1999) KCNE1-like gene is deleted in AMME contiguous gene syndrome: identification and characterization of the human and mouse homologs. Genomics 60, 251–257.
Tai, K. and Goldstein, S. (1998) The conduction pore of a cardiac potassium channel. Nature 391, 605–608.
Melman, Y. F., Um, S. Y., Krumerman, A., Kagan, A., and McDonald, T. V., (2004) KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron 42, 927–937.
Chen, H., Sesti, F., and Goldstein, S. A., (2003) Pore- and state-dependent cadmium block of I(Ks) channels formed with MinK-55C and wild-type KCNQ1 subunits. Biophys. J. 84, 3679–3689.
Sesti, F. and Goldstein, S. A., (1998) Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J. Gen. Physiol. 112, 651–663.
Sesti, F., Tai, K. K., and Goldstein, S. A., (2000) MinK endows the I(Ks) potassium channel pore with sensitivity to internal tetraethylammonium. Biophys. J. 79, 1369–1378.
Yang, Y., and Sigworth, F. J., (1998) Single-Channel Properties of IKs Potassium Channels. J. Gen. Physiol. 112, 665–678.
Pusch, M., (1998) Increase of the single-channel conductance of KvLQT1 potassium channels induced by the association with minK. Pflugers Arch. 437, 172–174.
Barhanin, J., Lesage, F., Guillemare, E., Fink, M., Lazdunski, M., and Romey, G., (1996) K(V)LQT1 and IsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384, 78–80.
Sanguinetti, M., Curran, M., Zou, A., et al. (1996) Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384, 80–83.
Marx, S. O., Kurokawa, J., Reiken, S., et al. (2002) Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295, 496–499.
Kurokawa, J., Chen, L., and Kass, R. S. (2003) Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc. Natl. Acad. Sci. USA 100, 2122–2127.
McDonald, T., Yu, Z., Ming, Z., et al. (1997) A minK-HERG complex regulates the cardiac potassium current I(Kr). Nature 388, 289–292.
Lewis, A., McCrossan, Z. A., and Abbott, G. W., (2004) MinK, MiRP1, and MiRP2 diversify Kv3.1 and Kv3.2 potassium channel gating. J. Biol. Chem. 279, 7884–7892.
Tinel, N., Diochot, S., Lauritzen, I., Barhanin, J., Lazdunski, M., and Borsotto, M., (2000) M-type KCNQ2-KCNQ3 potassium channels are modulated by the KCNE2 subunit. FEBS Lett. 480, 137–141.
Tinel, N., Diochot, S., Borsotto, M., Lazdunski, M., and Barhanin, J., (2000) KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J. 19, 6326–6330.
Yu, H., Wu, J., Potapova, I. et al. (2001) MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ. Res. 88, E84-E87.
Zhang, M., Jiang, M., and Tseng, G., (2001) minK-related peptide 1 associates with Kv4.2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel?. Circ. Res. 88, 1012–1019.
Decher, N., Bundis, F., Vajna, R., and Steinmeyer, K., (2003) KCNE2 modulates current amplitudes and activation kinetics of HCN4: influence of KCNE family members on HCN4 currents. Pflugers Arch. 446, 633–640.
Proenza C. Angoli, D., Agranovich, E., Macri, V., and Accili, E. A., (2002) J. Biol. Chem. 277, 5101–5109.
Schroeder, B., Waldegger, S., Fehr, S., et al. (2000) A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403, 196–199.
Abbott, G., Butler, M., Bendahhou, S., Dalaks, M., Ptacek, L., and Goldstein, S., (2001) MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell 104, 217–231.
McCrossan, Z. A., Lewis, A., Panaghie, G., et al., (2003) MinK-related peptide 2 modulates Kv2.1 and Kv3.1 potassium channels in mammalian brain. J. Neurosci. 23, 8077–8091.
Angelo, K., Jespersen, T., Grunnet, M., Nielsen, M. S., Klaerke, D. A., and Olesen, S. P., (2002) KCNE5 induces time- and voltage-dependent modulation of the KCNQ1 current. Biophys. J. 83, 1997–2006.
Grunnet, M., Rasmussen, H. B., Hay-Schmidt, A., et al. (2003) KCNE4 is an inhibitory subunit to Kv1.1 and Kv1.3 potassium channels. Biophys. J. 85, 1525–1537.
Splawski, I., Tristani-Firouzi, M., Lehmann, M. H., Sanguinetti, M. C., and Keating, M. T., (1997) Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat. Genet. 17, 338–340.
Sesti, F., Abbott, G. W., Wei, J., et al. (2000) A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc. Natl. Acad. Sci. USA 97, 10613–10618.
Splawski, I., Shen, J., Timothy, K. W., et al. (2000) Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102, 1178–1185.
Abbott, G. W., and Goldstein, S. A., (2002) Disease-associated mutations in KCNE potassium channel subunits (MiRPs) reveal promiscuous disruption of multiple currents and conservation of mechanism. FASEB J. 16, 390–400.
Gordon, E., Roepke, T. K., and Abbott, G. W., (2006) Endogenous KCNE subunits govern Kv2.1 K+ channel activation kinetics in Xenopus oocyte studies. Biophys. J. 90, 1223–1231.
Takumi, T., Moriyoshi, K., Aramori, I., et al. (1991) Alteration of channel activities and gating by mutations of slow ISK potassium channel. J. Biol. Chem. 266, 22,192–22,198.
Goldstein, S. A., and Miller, C., (1991) Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron 7, 403–408.
Melman, Y. F., Domenech, A., de la Luna, S., and McDonald, T. V. (2001) Structural determinants of KvLQT1 control by the KCNE family of proteins. J. Biol. Chem. 276, 6439–6444.
Melman, Y. F., Krumerman, A., and McDonald, T. V., (2002) A single transmembrane site in the KCNE-encoded proteins controls the specificity of KvLQT1 channel gating. J. Biol. Chem. 277, 25,187–25,194.
Cui, J., Kagan, A., Qin, D., Mathew, J., Melman, Y. F., and McDonald, T. V., (2001) Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J. Biol. Chem. 276, 17,244–17,251.
Gage, S., and Kobertz, W., (2004) KCNE3 truncation mutants reveal a bipartite modulation of KCNQ1 K+ channels. J. Gen. Physiol. 124, 759–771.
Park, K. H., Kwok, S. M., Sharon, C., Berga, R., and Sesti, F. (2003) N-Glycosylation-dependent block is a novel mechanism for drug-induced cardiac arrhythmia. FASEB J. 17, 2308–2309.
Lu, Y., Mahaut-Smith, M. P., Huang, C. L., and Vandenberg, J. I., (2003) Mutant MiRP1 subunits modulate HERG K+ channel gating: a mechanism for pro-arrhythmia in long QT syndrome type 6. J. Physiol. 551, 253–262.
Isbrandt, D., Friederich, P., Solth, A., et al. (2002) Identification and functional characterization of a novel KCNE2 (MiRP1) mutation that alters HERG channel kinetics. J. Mol. Med. 80, 524–532.
Yang, Y., Xia, M., Jin, Q., et al. (2004) Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am. J. Hum. Genet. 75, 899–905.
Panaghie, G., Tai, K. K., and Abbott, G. W., (2006) Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J. Physiol. 570, 455–467.
Bianchi, L., Shen, Z., Dennis, A., et al. (1999) Human Mol. Genet. 8, 1499–1507.
Tzounopoulos, T., Guy, H. R., Durell, S., Adelman, J. P., and Maylie, J., (1995) min K channels form by assembly of at least 14 subunits. Proc. Natl. Acad. Sci. USA 92, 9593–9597.
Wang, K. W., and Goldstein, S. A., (1995) Subunit composition of minK potassium channels. Neuron 14, 1303–1309.
Chen, H., Kim, L. A., Rajan, S., Xu, S., and Goldstein, S. A. (2003) Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron 40, 15–23.
Tai, K. K., Wang, K. W., and Goldstein, S. A., (1997) MinK potassium channels are heteromultimeric complexes. J. Biol. Chem. 272, 1654–1658.
Wang, K. W., Tai, K. K., and Goldstein, S. A., (1996) MinK residues line a potassium channel pore. Neuron 16, 571–577.
Tapper, A., and George, A., Jr., (2000) MinK subdomains that mediate modulation of and association with KvLQT1. J. Gen. Physiol. 116, 379–390.
Kurokawa, J., Motoike, H., and Kass, R., (2001) TEA(+)-sensitive KCNQ1 constructs reveal pore-independent access to KCNE1 in assembled I(Ks) channels. J. Gen. Physiol. 117, 43–52.
Cai, S. Q., Hernandez, L., Wang, Y., Park, K. H. and Sesti, F. (2005) MPS-1 is a K+ channel beta-subunit and a serine/threonine kinase. Nat. Neurosci. 8, 1503–1509.
Ryazanov, A. G., Pavur, K. S., and Dorovkov, M. V., (1999) Alpha-kinases: a new class of protein kinases with a novel catalytic domain. Curr. Biol. 9, R43-R45.
Runnels, L. W., Yue, L., and Clapham, D. E., (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047.
Drennan, D., and Ryazanov, A. G. (2004) Alpha-kinases: analysis of the family and comparison with conventional protein kinases. Prog. Biophys. Mol. Biol. 85, 1–32.
Stein, L. D., Bao, Z., Blasiar, D., et al. (2003) The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1, E45.
Bargmann, C., and Mori, I., (1997) Chemotaxis and thermotaxis. In C. elegans II (Riddle D. L., Meyer B. J., Priess J. R., eds.) Vol. 1, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp. 717–737.
Heitzmann, D., Grahammer, F., von Hahn, T., et al. (2004) Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J. Physiol. 561, 547–557.
Tobin, D., Madsen, D., Kahn-Kirby, A., et al. (2002) Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35, 307–318.
O'Hagan, R., Chalfie, M., and Goodman, M. B., (2005) The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8, 43–50.
Lundquist, A. L., Manderfield, L. J., Vanoye, C. G., et al. (2005) Expression of multiple KCNE genes in human heart may enable variable modulation of I(Ks). J. Mol. Cell. Cardiol. 38, 277–287.
Finley, M. R., Li, Y., Hua, F., et al. (2002) Expression and coassociation of ERG1, KCNQ1 and KCNE1 potassium channel proteins in horse heart. Am. J. Physiol. Heart Circ. Physiol. 283, H126–138.
Chun, K., Koenen, M., Katus, H., and Zehelein, J. (2004) Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp. Mol. Med. 36, 367–371.
Jiang, M., Zhang, M., Tang, D. G., et al. (2004) KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation 109, 1783–1788.
Christensen, M., Estevez, A., Yin, X., et al. (2002) A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron 33, 503–514.
Goodman, M., Hall, D., Avery, L., and Lockery, S., (1998) Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron 20, 763–772.
Pierce-Shimomura, J., Faumont, S., Gaston, M., Pearson, B., and Lockery, S., (2001) The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410, 694–698.
Connor, J. A., and Stevens, C. F., (1971) Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J. Physiol. 213, 1–19.
Connor, J. A., and Stevens, C. F., (1971) Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J. Physiol 213, 21–30.
Grahammer, F., Herling, A. W., Lang, H. J., et al. (2001) The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 120, 1363–1371.
Dedek, K., and Waldegger, S., (2001) Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 442, 896–902.
Cui, J., Melman, Y., Palma, E., Fishman, G., and McDonald, T., (2000) Cyclic AMP regulates the HERG K(+) channel by dual pathways. Curr. Biol. 10, 671–674.
Qu, J., Kryukova, Y., Potapova, I. A., et al. (2004) MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J. Biol. Chem. 279, 43,497–43,502.
Grunnet, M., Jespersen, T., Rasmussen, H. B., et al. (2002) KCNE4 is an inhibitory subunit to the KCNQ1 channel. J. Physiol. 542, 119–130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, SQ., Park, K.H. & Sesti, F. An evolutionarily conserved family of accessory subunits of K+ channels. Cell Biochem Biophys 46, 91–99 (2006). https://doi.org/10.1385/CBB:46:1:91
Issue Date:
DOI: https://doi.org/10.1385/CBB:46:1:91